Method for synthesizing and preparing pradaxa formamidine intermediates
A dabigatran etexilate and synthetic method technology, applied in the direction of organic chemistry, etc., can solve the problems that alcoholysis impurities cannot be effectively removed, ammonolysis impurities have no refining effect, and impurities cannot be effectively removed, so as to shorten the time of dangerous operations and save energy. Solvent, reduce the effect of equipment pressure resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
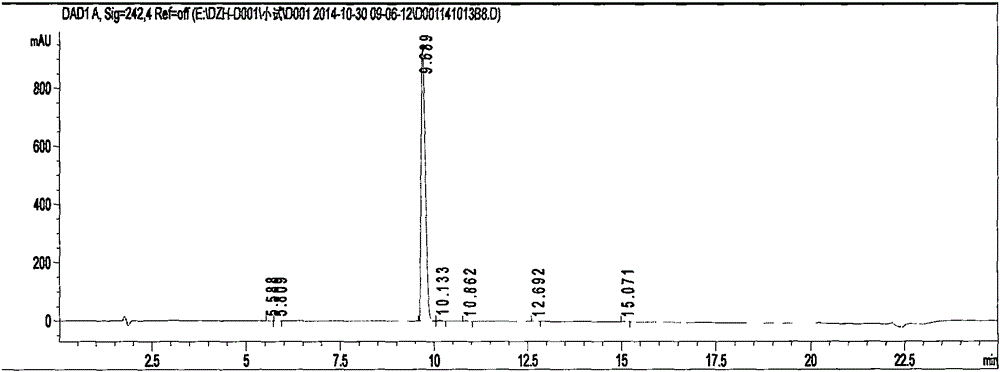
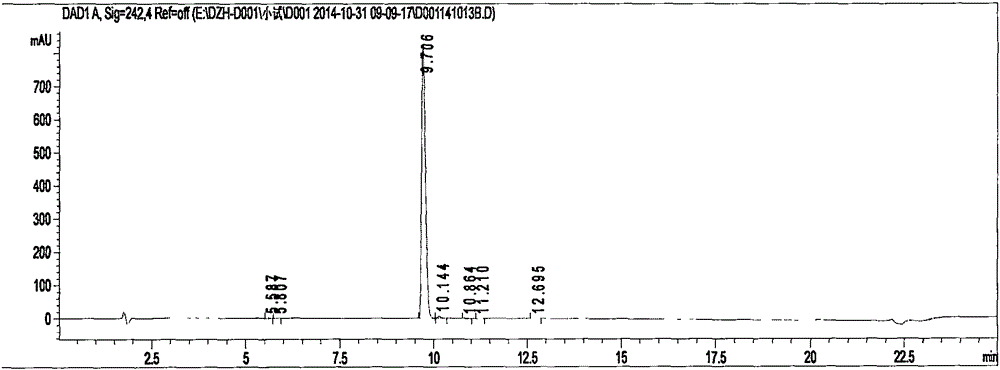
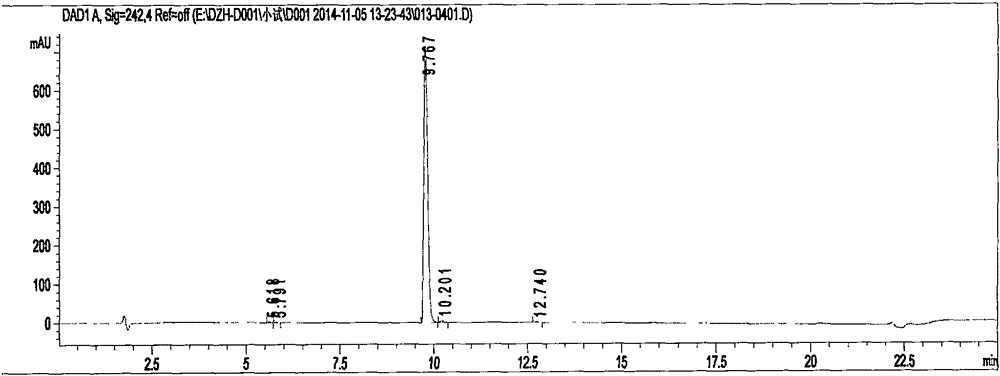
Image
Examples
Embodiment 1
[0040] In a 5L dry three-neck round bottom reaction flask, add 3-(3-(2-(4-cyanoanilino)acetamido)4-(methylamino)-N-(pyridin-2-yl)benzene Add 300 g of formamido)-ethyl propionate, add 2.4 L of absolute ethanol, stir and cool down to -10°C. Pass dry hydrogen chloride gas, control the solution temperature below 35°C, until the hydrogen chloride in the solution reaches saturation. Stop feeding hydrogen chloride gas, and raise the temperature to 35°C. After 3.0 hours, HPLC monitoring showed that the remaining raw materials were about 8.2%, and the temperature was raised to 40° C., and excess hydrogen chloride gas was distilled under reduced pressure.
[0041] Add 2.4L of absolute ethanol to the reaction bottle, continue to cool down to 5°C, and pass ammonia gas under stirring to control the solution temperature at 5-15°C. It was detected that the pH of the reaction solution was 9.5, the feeding of ammonia gas was stopped, and the temperature was raised to 30°C. HPLC monitors, an...
Embodiment 8
[0047] In a 50L dry glass double-layer reactor, add 3-(3-(2-(4-cyanoanilino)acetamido)4-(methylamino)-N-(pyridin-2-yl)benzyl Amino)-ethyl propionate 1kg, add 6L absolute ethanol, stir and cool down to -10°C. Start to feed dry hydrogen chloride gas, and control the temperature of the solution to be lower than 30°C until the hydrogen chloride in the solution reaches saturation. Stop feeding hydrogen chloride gas, and raise the temperature to 30°C. After 3.0 hours, HPLC monitoring showed that the remaining raw materials were about 8.9%, and the temperature was raised to 40° C., and excess hydrogen chloride gas was distilled under reduced pressure.
[0048] Add 6L of absolute ethanol to the reaction bottle, continue to cool down to -5°C, and pass ammonia gas under stirring to control the solution temperature at 25-30°C. It was detected that the pH of the reaction solution was 9.5, the feeding of ammonia gas was stopped, and the temperature was raised to 30°C. HPLC monitors, and...
Embodiment 9
[0051] In a 50L dry glass double-layer reactor, add 3-(3-(2-(4-cyanoanilino)acetamido)4-(methylamino)-N-(pyridin-2-yl)benzyl Amino)-ethyl propionate 3kg, add 18L absolute ethanol, stir and cool down to -10°C. Start to feed dry hydrogen chloride gas, and control the temperature of the solution to be lower than 30°C until the hydrogen chloride in the solution reaches saturation. Stop feeding hydrogen chloride gas, and raise the temperature to 30°C. After 3.0 hours, HPLC monitoring showed that the remaining raw materials were about 8.8%, and the temperature was raised to 40° C., and excess hydrogen chloride gas was distilled under reduced pressure.
[0052] Add 18L of absolute ethanol to the reaction flask, continue to cool down to -5°C, and pass ammonia gas under stirring to control the solution temperature at 25-30°C. It was detected that the pH of the reaction solution was 9.5, the feeding of ammonia gas was stopped, and the temperature was raised to 30°C. HPLC monitors, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com