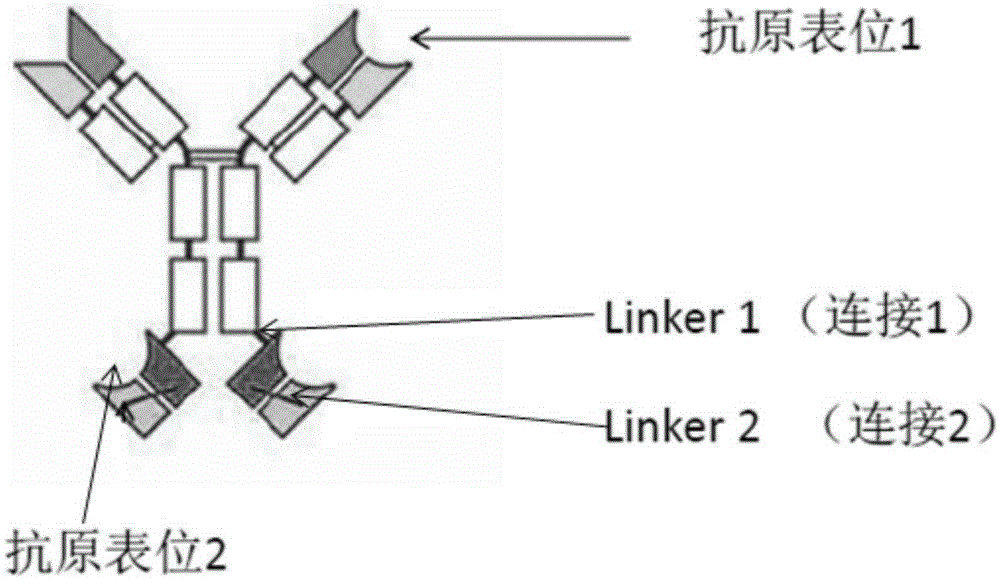
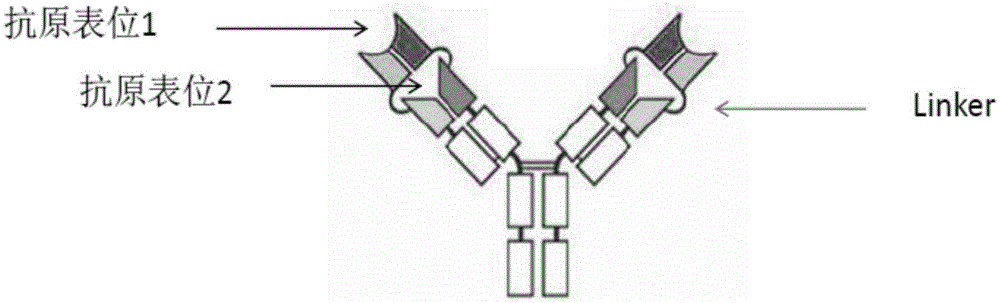
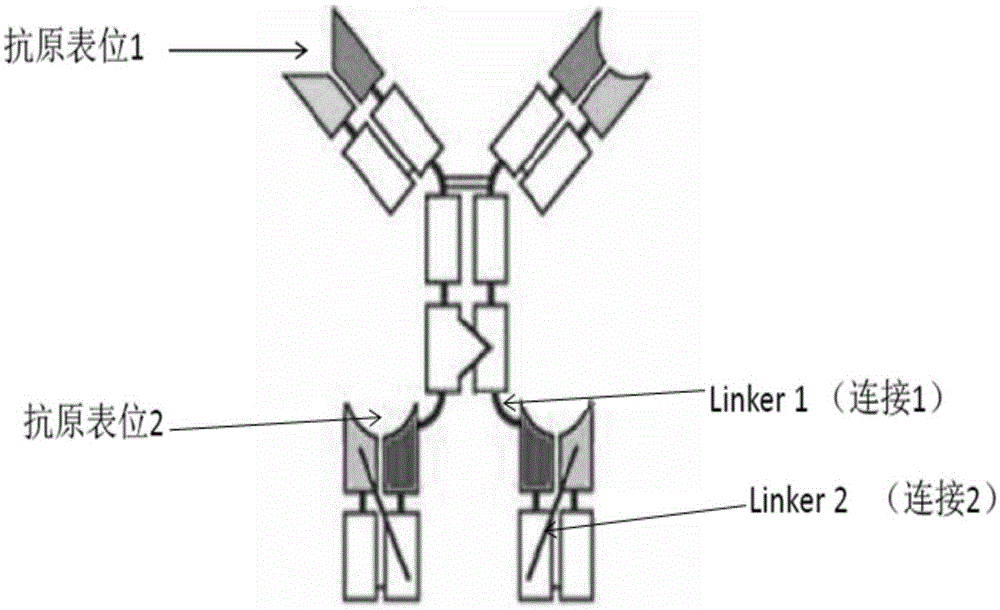
Bifunctional antibodies and uses thereof
A bifunctional antibody and multipurpose technology, applied in the fields of antibodies, anti-inflammatory agents, digestive system, etc., can solve the problems of limited efficacy of monoclonal antibodies
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: Protein preparation of bifunctional antibodies BIAU003, BIAU022 and BIAU023 of the present invention
[0025] According to the heavy chain and light chain amino acid sequences of bifunctional antibodies BIAU003, BIAU022 and BIAU023, cDNAs of heavy chains and light chains of bifunctional antibodies BIAU003, BIAU022 and BIAU023 were artificially synthesized, respectively. All cDNAs are codon-optimized for expression in mammalian cells. The synthesized cDNAs were cloned into pcDNA3.1 plasmids respectively, and the plasmids were constructed correctly by sequencing. The sequenced plasmid was transformed into the TOP10 strain, and a single colony was picked and inoculated into 0.5 liters of LB liquid medium. When the OD600 was 0.8, the bacteria were collected by centrifugation, and the plasmid was extracted with a plasmid extraction kit (purchased from Qiagen). Co-transfect the plasmid containing the light chain of the bifunctional antibody BIAU003 identified corr...
Embodiment 2
[0026] Example 2: SPR determination of the antigen-binding ability of the bifunctional antibodies BIAU003, BIAU022 and BIAU023 of the present invention
[0027] The affinity and binding kinetics of p40 and TNF-α (purchased from GE) were analyzed by SPR. p40 or TNF-[alpha] was covalently attached to the chip via primary amines (carboxymethyl dextran-coated chip) using standard amine coupling chemistry and chips provided by GE. The recombinant fusion protein was covalently coupled to biotin using a biotin labeling kit (Pierce Company), and then flowed through an avidin-labeled SA chip (purchased from GE Company), so that the reaction value RU reached 450. Association and dissociation constants were measured by flowing antibodies in PBS buffer at concentrations of 0.01, 0.03, 0.09, 0.27 μΜ and a flow rate of 50 μl / min. Antigen-antibody binding kinetics were followed for 3 minutes and dissociation kinetics were followed for 10 minutes. The binding and dissociation curves were fi...
Embodiment 3
[0032] Example 3: Competition ELISA method to determine that the bifunctional antibodies BIAU003, BIAU022 and BIAU023 of the present invention compete with TNF-α receptors for binding to antigen TNF-α
[0033] Coat the ELISA plate with TNF-α-mFc, block with 1% BSA, mix different concentrations of antibodies BIAU003, BIAU022, BIAU023 with TNF-α receptor-hFc, incubate at 37°C, add the enzyme-labeled secondary antibody and incubate at 37°C 30 minutes. The absorbance at 450 nm was detected on a microplate reader. The results of the binding of the bifunctional antibody to the antigen TNF-α show that the bifunctional antibodies BIAU003, BIAU022, and BIAU023 of the present invention can all effectively compete with the TNF-α receptor for binding to the TNF-α protein, and the binding efficiency is dose-dependent. Through the analysis of the competition ELISA results of the combined bifunctional antibodies, the curve simulation bifunctional antibodies BIAU003, BIAU022, and BIAU023 ant...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com