Method for separating copper, cobalt and manganese from cupric chloride manganese-cobalt-calcium-zinc impurity removal solution
A technology of copper, manganese, cobalt, calcium, zinc and copper, cobalt, and manganese chloride, which is applied in crucible furnaces, electric furnaces, drum furnaces, etc., to achieve the effects of low cost, short process and high metal yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
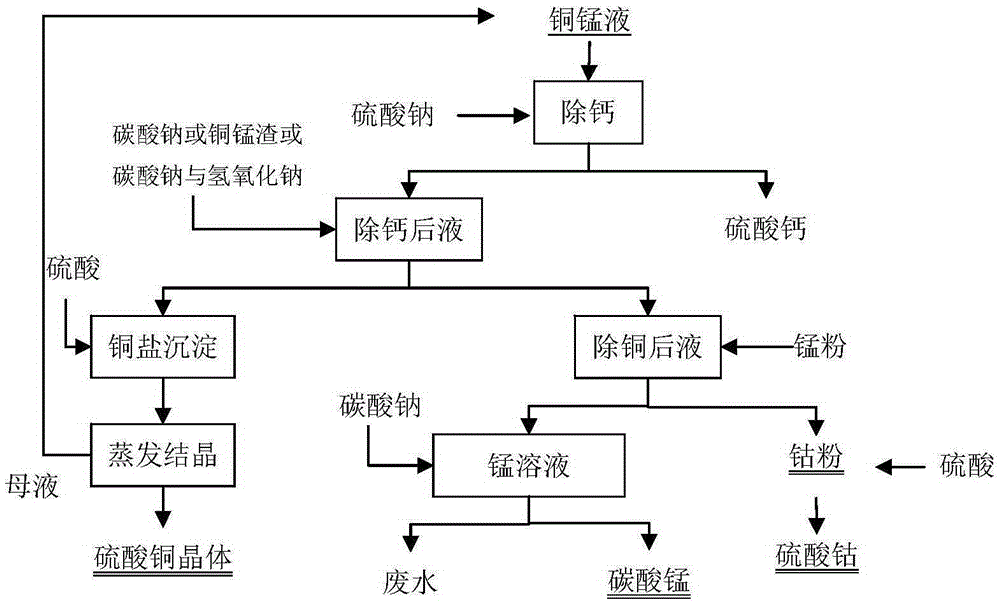
[0025] A method for separating copper-cobalt-manganese from the copper-manganese-cobalt-calcium-zinc impurity removal solution produced by the cobalt production system is operated according to the following steps:
[0026] Take 200mL of copper, manganese, cobalt, calcium and zinc chloride solution, its composition is copper 35.1g / L, manganese 122g / L, zinc 7.5g / L, calcium 12.2g / l, cobalt 13.8g / L. Add 18g of anhydrous sodium sulfate, stir and react for 30min, and remove calcium sulfate precipitate by suction filtration. Heat the solution to 70°C, add 21% sodium carbonate solution dropwise to pH 4.7, filter, wash the filter cake with a small amount of pure water, add 20mL of pure water to make slurry, add 98% concentrated sulfuric acid to dissolve, After evaporation and crystallization, 20.6 g of copper sulfate crystals were obtained. The direct yield of primary crystallized copper is 74.9%. The overall copper recovery was 97.5%.
[0027] Heat the solution after copper removal...
Embodiment 2
[0030] Take 200mL of copper, manganese, cobalt, calcium and zinc chloride solution, its composition is copper 43.8g / L, manganese 79.6g / L, zinc 3.9g / L, calcium 9.1g / l, cobalt 2.3g / L. Add 11 g of anhydrous sodium sulfate, stir the reaction for 30 minutes, and remove calcium sulfate precipitate by suction filtration. Heat the solution to 70°C, add 21% sodium carbonate solution dropwise to pH 5.1, filter, wash the filter cake with a small amount of pure water, add 20mL pure water to make slurry, add 98% concentrated sulfuric acid to dissolve, After evaporation and crystallization, 27.8 g of copper sulfate crystals were obtained. The direct recovery rate of primary crystallized copper is 81%. The overall copper recovery was 98.6%.
[0031] Heat the solution after copper removal to 70°C, add 0.9g of manganese powder, the cobalt ions are reduced to cobalt powder, filter and separate the cobalt powder, dissolve the cobalt powder with sulfuric acid to obtain a cobalt sulfate solution...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com