Preparation method and application of alkaline copper chloride crystal
A copper chloride and basic technology, applied in the field of basic copper chloride preparation, can solve the problems of strong oxidation, hardening and corrosion processing, damage to vitamins, etc. low effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example
[0043] The raw material used in all the following examples, namely clinocopperite, was prepared according to the preparation method including the following steps:
[0044] A1: Prepare 15% ammonium chloride aqueous solution, copper chloride aqueous solution (in which the copper concentration is 200g / L) and NH 3 Ammonia aqueous solution with a mass concentration of 12%;
[0045] A2: Preheat the ammonium chloride aqueous solution, the copper chloride aqueous solution and the ammonia aqueous solution respectively, so that the temperature of these three solutions are all 85°C;
[0046] A3: Add the ammonium chloride aqueous solution to the reactor, the adding amount is 50% of the total volume of the materials at the end of the reaction, and then add the copper chloride aqueous solution and the ammonia aqueous solution while stirring to control the pH of the reaction system to 5.5 , Reaction feeding time is 25min, copper chloride in copper chloride aqueous solution: NH in aqueous ammonia so...
Embodiment 1
[0048] Add 1,000 kg of feed grade chlorocopperite and 4,000 liters of water into the reactor to form a reaction system, stir while passing steam, heat the reaction system at 75°C, and add aqueous ammonia solution to control the pH of the reaction system 5. Control the reaction time to 120min to generate parachlorocopperite precipitation;
[0049] The generated parachlorocopperite precipitate is filtered, washed with tap water, dried at 105°C, and sieved with a sieve with a diameter of 45μm to obtain a particle size greater than 45μm and a weight of 992 kg (that is, the conversion rate from raw material to product Up to about 99%) dark green parachlorocopperite powder, named P1.
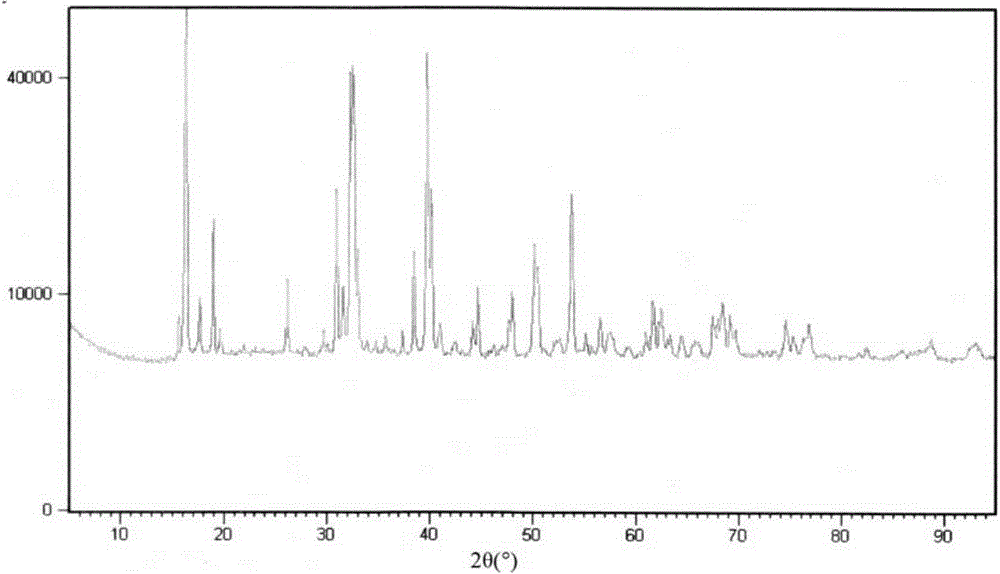
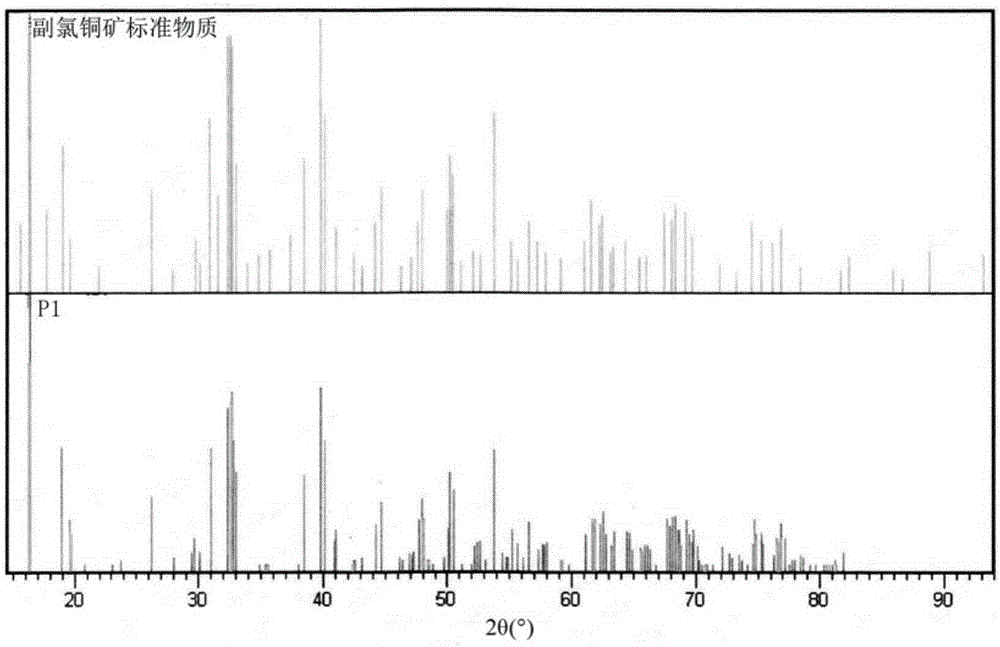
[0050] Such as figure 1 , figure 2 As shown, it was tested by the National 863 Shenzhen Material Surface Analysis and Testing Center with the Rigaku Dmax / 2000 automatic polycrystalline powder X-ray diffractometer: the structural formula of the basic copper chloride is Cu 2 (OH) 3 Cl (Paratacamite, parach...
Embodiment 2
[0053] Add 1200 kg of feed grade chlorocopperite and 4000 liters of water into the reactor to form a reaction system, stir while passing steam, heat the reaction system at 50°C, and add aqueous ammonia solution to control the pH of the reaction system 7. Control the reaction time to 300min to generate parachlorocopperite precipitation;
[0054] The generated parachlorocopperite precipitate is filtered, washed with tap water, dried at 105°C, and sieved with a mesh with a diameter of 45μm to obtain a dark green parachlorocopperite powder with a particle size greater than 45μm and a weight of 1190 kg, named For P2.
[0055] Such as figure 1 , figure 2 As shown, it was tested by the National 863 Shenzhen Material Surface Analysis and Testing Center with the Rigaku Dmax / 2000 automatic polycrystalline powder X-ray diffractometer: the structural formula of the basic copper chloride is Cu 2 (OH) 3 Cl (Paratacamite, parachlorocopperite) belongs to the monoclinic system (monoclinic), the s...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com