Argatroban compound
An argatroban and compound technology, applied in the field of monohydrate crystal form, can solve problems such as restricting the development of argatroban-related preparations, affecting the quality of raw materials, etc., and achieving improved bioavailability, safety, and good water solubility , the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] The preparation of embodiment 1 argatroban compound
[0039] Weigh 50g of argatroban, add 100mL of methanol, 100mL of ethanol and 1800mL of water, heat up to 100°C, stir to dissolve, then naturally cool to room temperature for 12 hours, suction filter, wash, and dry to obtain 48.4g of white powder Argatroban monohydrate crystals with a yield of 96.8%. Mass spectrum showed that its ESIm / z: 509.3, 21(R): 21(S) = 64.7: 35.3 (HPLC method), water content 3.54% (Karl Fischer method).
[0040] Elemental analysis:
[0041]
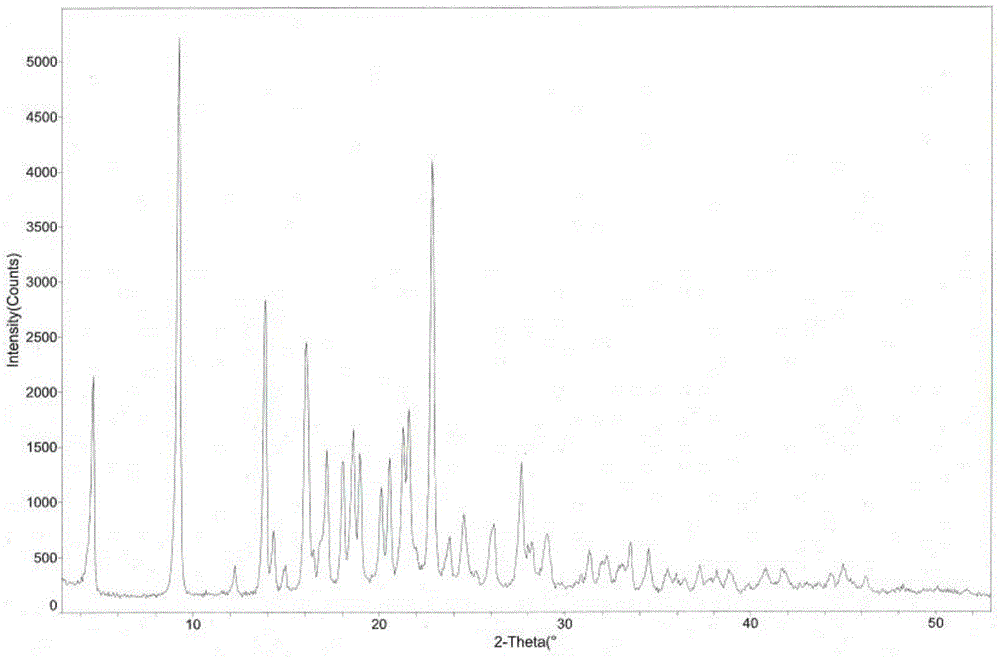
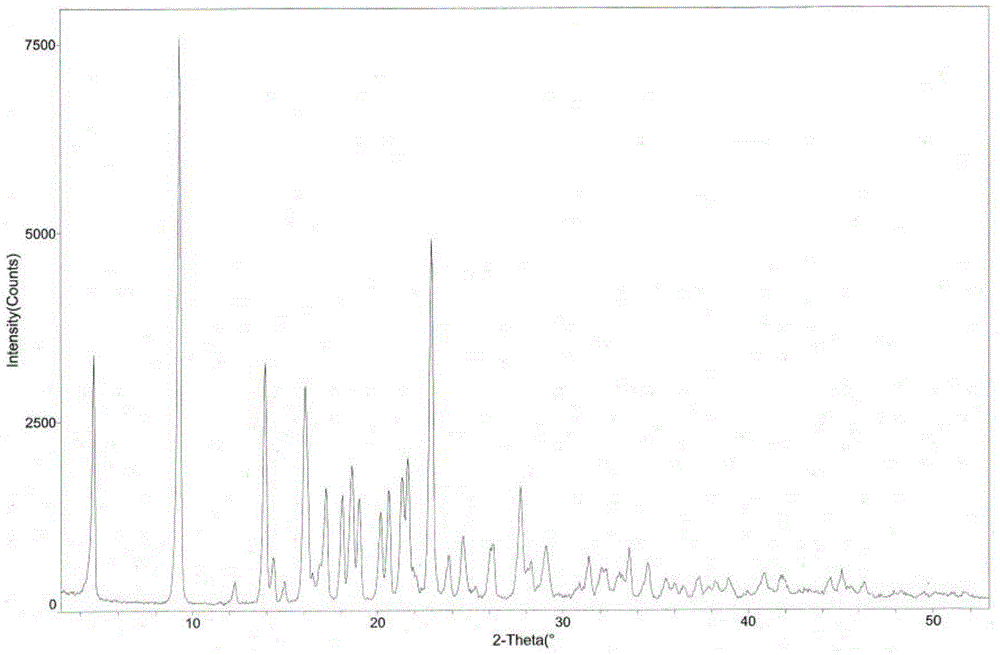
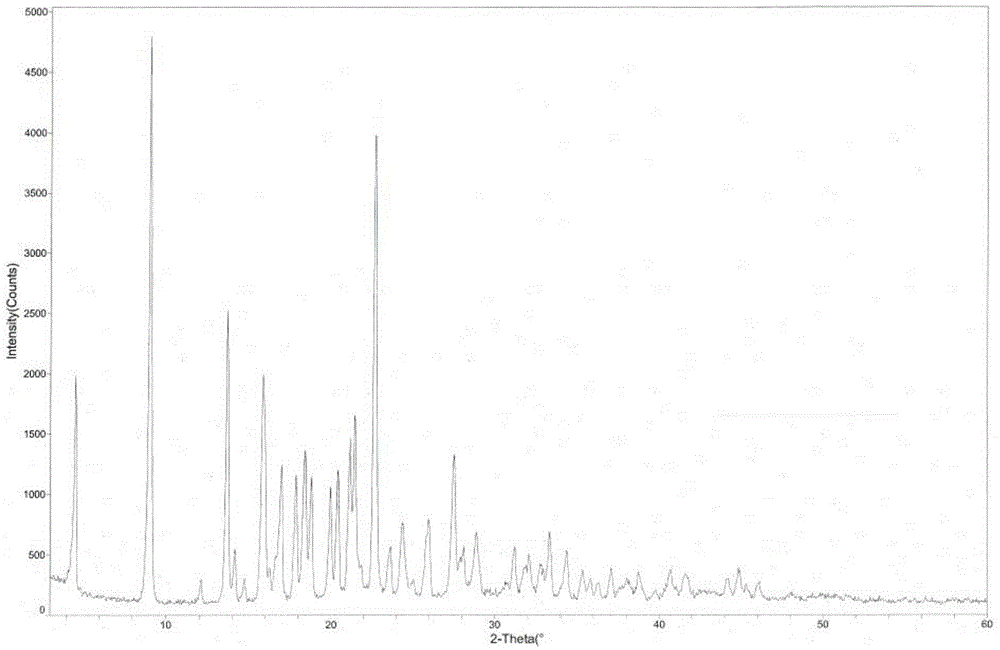
[0042] The measured melting point of argatroban monohydrate crystals is 185.5-186.5°C. Using CuKα radiation, the X-ray powder diffraction pattern of the crystal is measured in figure 1 , and the diffraction related data are shown in Table 1 (2θ measurement error is ±0.1).
[0043] Table 1 X-ray powder diffraction data of argatroban monohydrate crystal
[0044]
[0045]
[0046] By comparison with the existing literature, the 2θ diffraction ang...
Embodiment 2
[0047] The preparation of embodiment 2 argatroban compound
[0048] Weigh 50g of argatroban, add 200mL of methanol, 100mL of ethanol and 1000mL of water, heat up to 85°C, stir to dissolve, then naturally cool to room temperature for 2 hours, suction filter, wash, and dry to obtain 48.2g of white powder Argatroban monohydrate crystals with a yield of 96.4%. The structural analysis results and X-ray powder diffraction patterns of the obtained product are not significantly different from those in Example 1.
Embodiment 3
[0049] The preparation of embodiment 3 argatroban compound
[0050] Weigh 50g of argatroban, add 100mL of methanol, 200mL of ethanol and 1200mL of water, heat up to 70°C, stir to dissolve, then naturally cool to room temperature for 2 hours, then place at 0±5°C for 8 hours, suction filter and wash , and dried to obtain 48.5g of white powdery argatroban monohydrate crystals, with a yield of 97.0%. The structural analysis results and X-ray powder diffraction patterns of the obtained product are not significantly different from those in Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com