Glutaminyl cyclase inhibitor
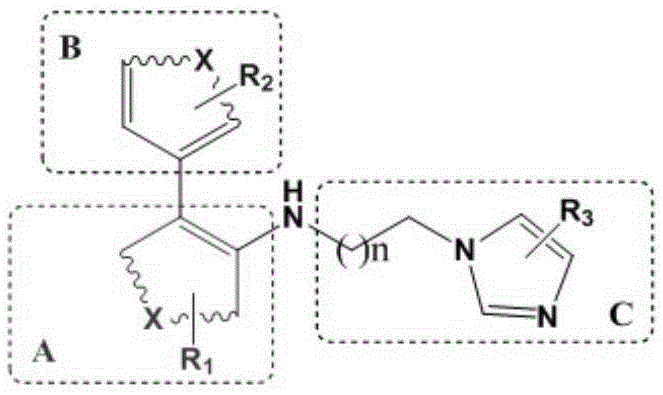
A glutaminyl cyclase and inhibitor technology, applied in the field of medicinal chemistry, can solve the problems of single parent structure, poor transmembrane performance, and poor water solubility, and achieve good water solubility, high druggability, and high activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: N-(3-(1H-imidazol-1-yl)propyl)-3',4'-dimethoxy-[1,1'-biphenyl]-2-amine (MQI-1 ), its synthetic route is as follows:
[0035]
[0036] a, 3',4'-dimethoxy-[1,1'-biphenyl]-2-amine: bromoaniline (5.81mmol, 1 equivalent), 3,4-dimethoxyphenylboronic acid (6.98 mmol, 1.2 equivalents) and [1,1'-bis(diphenylphosphino)ferrocene]dichloropalladium(II) dichloromethane complex (0.35mmol, 0.06 equivalents) were placed in a 50ml round bottom flask , respectively add 10ml of dioxane and 10ml of 2mol / LK 2 CO 3 Solution, 100 ° C, reflux for 3h. Add saturated NaCl solution to quench the reaction, cool to room temperature, extract three times with ethyl acetate, wash once with saturated NaCl solution after combining, anhydrous NaCl 2 SO 4 After drying, the product was collected by silica gel column chromatography with a yield of 90%.
[0037] b. N-(3-bromopropyl)-3',4'-dimethoxy-[1,1'-biphenyl]-2-amine: 3',4'-dimethoxy-[1 ,1'-biphenyl]-2-amine (872.32umol, 1 equivalent)...
Embodiment 2
[0039] Example 2: 4'-fluoro-N-(3-(4-methyl-1H-imidazol-1-yl)propyl)-[1,1'-biphenyl]-2-amine (MQI-31) The synthesis of its synthetic route is as follows:
[0040]
[0041] a, 4'-fluoro-[1,1'-biphenyl]-2-amine: bromoaniline (5.81mmol, 1 equivalent), 4-fluorophenylboronic acid (6.98mmol, 1.2 equivalents) and [1,1' -Bis (diphenylphosphine) ferrocene] palladium dichloride (II) dichloromethane complex (0.35mmol, 0.06 equivalent) is placed in 50ml round bottom flask, solvent is 10ml dioxane and 10ml2mol / LK 2 CO 3 solution, at 100°C, stirred for 3 h, added saturated NaCl solution to quench the reaction, extracted three times with ethyl acetate, combined and washed once with saturated NaCl solution, anhydrous NaCl 2 SO 4 After drying, the product was collected by silica gel column chromatography with a yield of 94%.
[0042] b. N-(3-bromopropyl)-4'-fluoro[1,1'-biphenyl]-2-amine: 4'-fluoro-[1,1'-biphenyl]-2-amine (534.15 umol, 1 equivalent) and 1,3-dibromopropane (3.74mmol, 7 ...
Embodiment 3
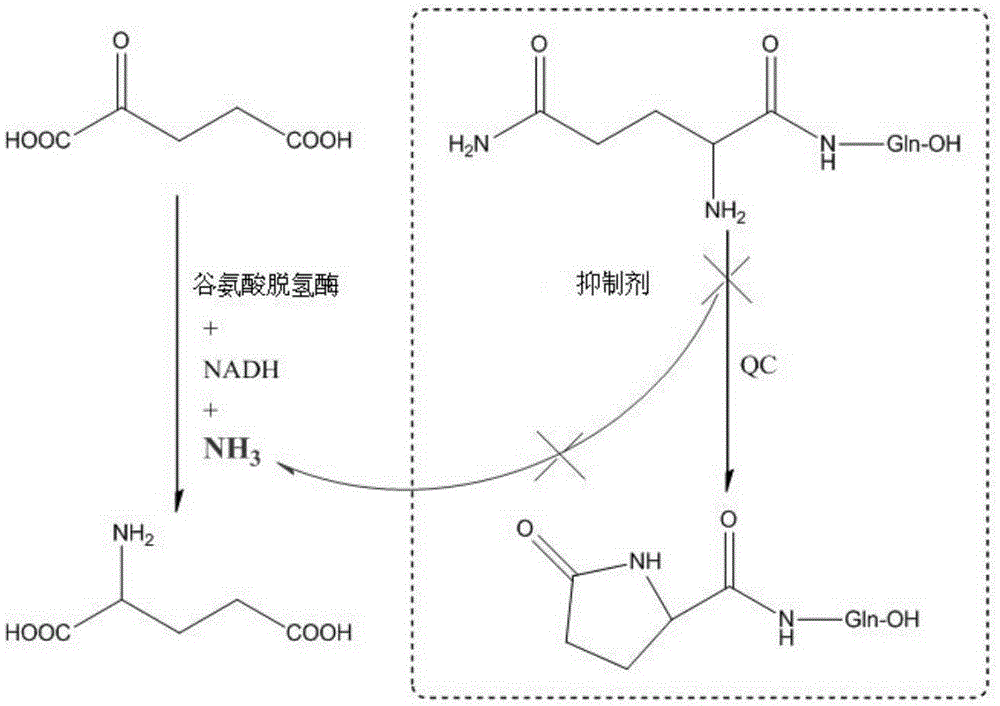
[0044] Embodiment 3: QC inhibitor is tested to QC enzyme inhibitory activity
[0045] The schematic diagram of the QC enzyme inhibition activity test principle is as follows: figure 1 shown. The enzyme activity test was carried out in a 96-well microtiter plate, using 200ul pH8.0 Tris buffer system: 0.3mM NADH, 2.0mM freshly prepared Gln-Gln, 14mM α-ketoglutarate, 30U / ml glutamate dehydrogenase, 50mM Tris, pH8 .0 buffer solution, and finally add 0.28 μM recombinant human QC protein and the mixture of different concentrations of inhibitors, shake for 30 seconds, and use a microplate reader to dynamically detect the change in the absorption value of NADH at 340nm wavelength within 15 minutes at 25°C, every 30s Once the data is collected, according to the test results, calculate the IC of the inhibition effect of the inhibitor on the QC enzyme activity 50 Value, the test result for different QC inhibitors is shown in table 1, wherein the structural formula of QC inhibitors is: ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com