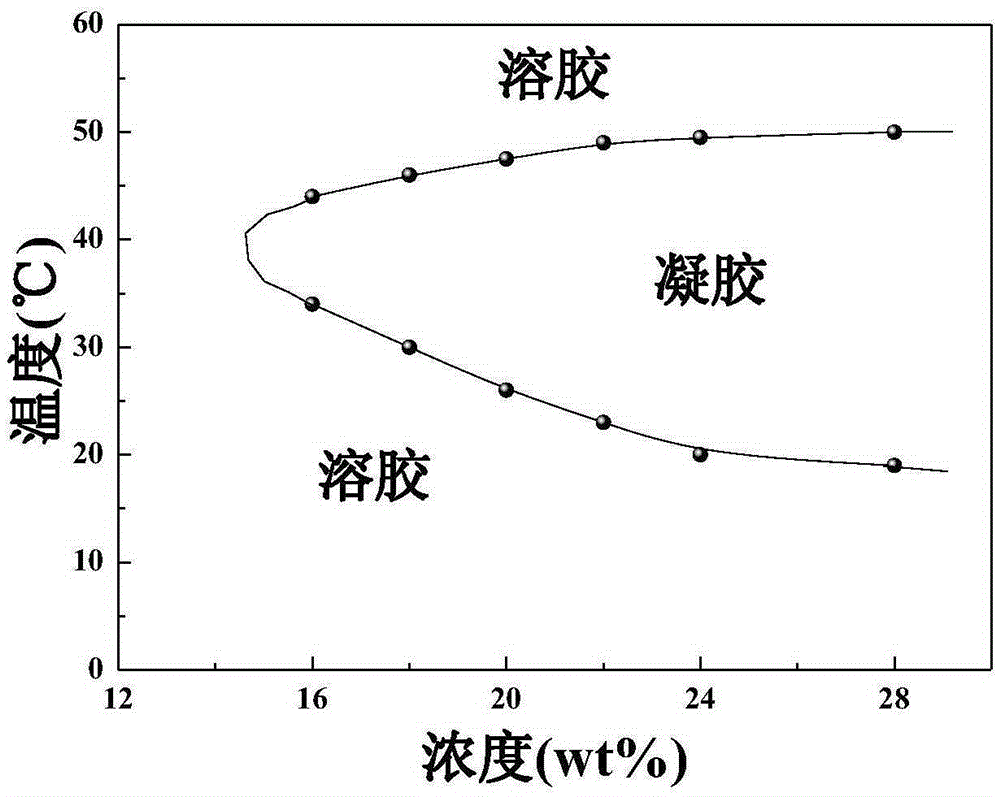
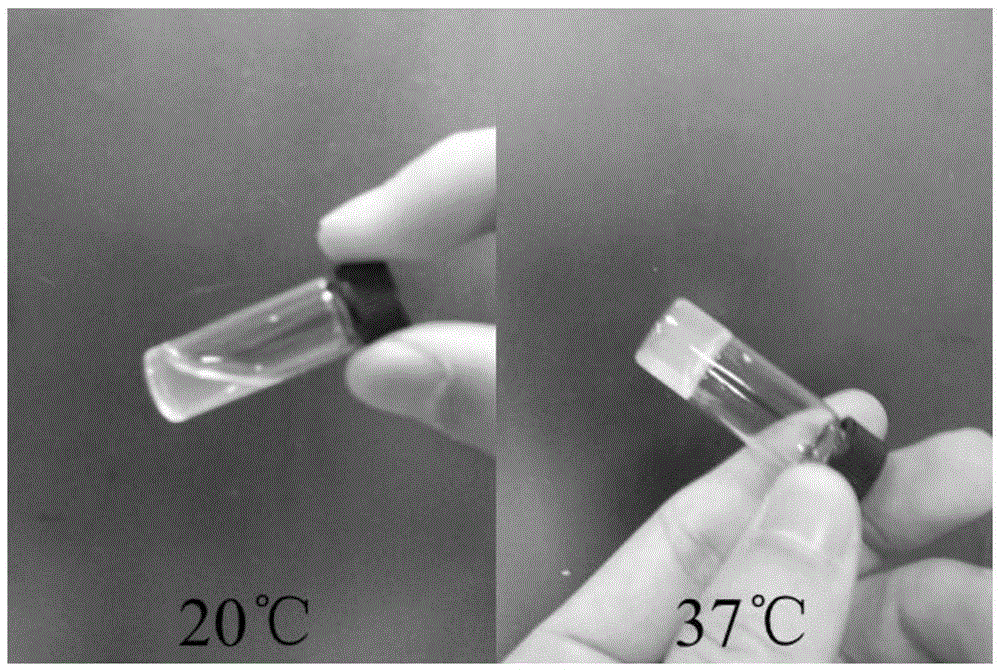
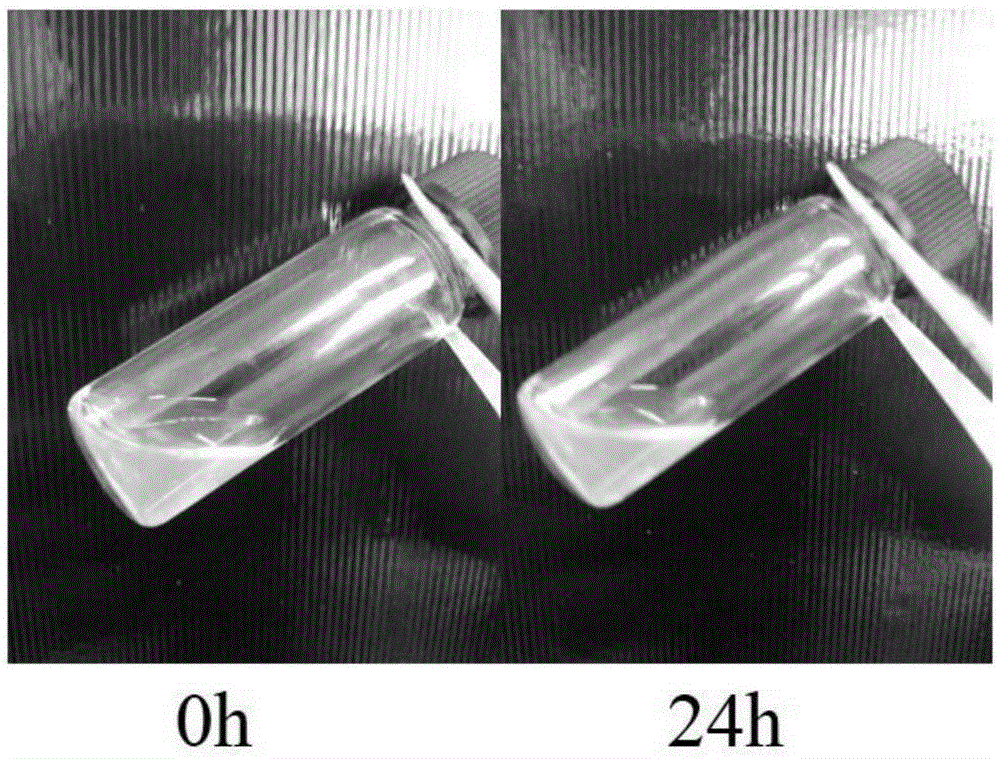
Injectable thermosensitive physical hydrogel and preparation method thereof
A technology of hydrogel and physical water, which is applied in the fields of pharmaceutical formulation, drug delivery, medical science, etc. It can solve the problems of excessive modulus, unsuitable for embedding cells, hindering application, etc., and achieve good biocompatibility and wide application Foreground, effect of improving stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Poly(caprolactone-co-gamma-benzyl carbamate caprolactone)-polyethylene glycol-poly(caprolactone-co-gamma-benzyl carbamate caprolactone) (P Synthesis of (CL-co-CABCL)-PEG-P(CL-co-CABCL))
[0033] In the state of vacuuming, bake a 10ml polymerization bottle (a glass bottle with a suction device that can be sealed after vacuuming) on a gas lamp for three times to fully remove water. Then feed argon, under the protection of argon, the polyethylene glycol (PEG, 1g) that molecular weight is 1500, caprolactone (CL, 1.2g) and gamma-benzyl carbamate caprolactone (CABCL, 0.05g) into the polymerization bottle. Then put the polymerization bottle in a constant temperature oil bath at 60°C to stir and evacuate for 2 hours to fully mix the raw materials and remove traces of toluene, water and other small molecular impurities that may exist in the raw materials. Next, the catalyst Sn(Oct) was added in an atmosphere protected by argon 2 (one-thousandth of the amount of m...
Embodiment 2
[0034] Example 2: Poly(caprolactone-co-γ-aminocaprolactone)-polyethylene glycol-poly(caprolactone-co-γ-aminocaprolactone) (P(CL-co-ACL)- Synthesis of PEG-P(CL-co-ACL))
[0035]1 g of P(CL-co-CABCL)-PEG-P(CL-co-CABCL) was completely dissolved in 25 mL of tetrahydrofuran, and 0.2 g of palladium on carbon (Pd / C, 10%) was added. The round bottom flask was bubbled three times with dry nitrogen, then hydrogen was continuously bubbled into the flask. After stirring at room temperature for 72 hours, the reaction was stopped, and the Pd / C was filtered out with a floxacin funnel, most of the tetrahydrofuran was removed by rotary evaporation, settled in excess ether, and vacuum-dried for 2 days to constant weight to obtain pure and dry poly(hexyl) Ester-copoly-γ-aminocaprolactone)-polyethylene glycol-poly(caprolactone-copoly-γ-aminocaprolactone) (P(CL-co-ACL)-PEG-P(CL-co- ACL)) polymers. It is 5200 by 1HNMR measurement copolymer relative molecular mass, and the content of the epsilon-...
Embodiment 3
[0036] Embodiment 3: Poly(caprolactone-copoly-4-methylcaprolactone)-polyethylene glycol-poly(caprolactone-copoly-4-methylcaprolactone) (P(CL-co-MeCL )-PEG-P(CL-co-MeCL)) Synthesis
[0037] Under vacuum, bake the 10ml polymerization bottle on the gas lamp three times to fully remove the water. Then pass through argon, under the protection of argon, the polyethylene glycol (PEG, 1g) that molecular weight is 1000, caprolactone (CL, 1.4g) and 4-methyl caprolactone (MCL, 0.2g) Add to the polymerization bottle. Then put the polymerization bottle in a constant temperature oil bath at 60°C to stir and evacuate for 2 hours to fully mix the raw materials and remove traces of toluene, water and other small molecular impurities that may exist in the raw materials. Next, the catalyst Sn(Oct) was added in an atmosphere protected by argon 2 (one-thousandth of the amount of monomer), vacuumize and seal the polymerization bottle. After reacting at 130° C. for 24 hours under sufficient magn...
PUM
| Property | Measurement | Unit |
|---|---|---|
| transition temperature | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com