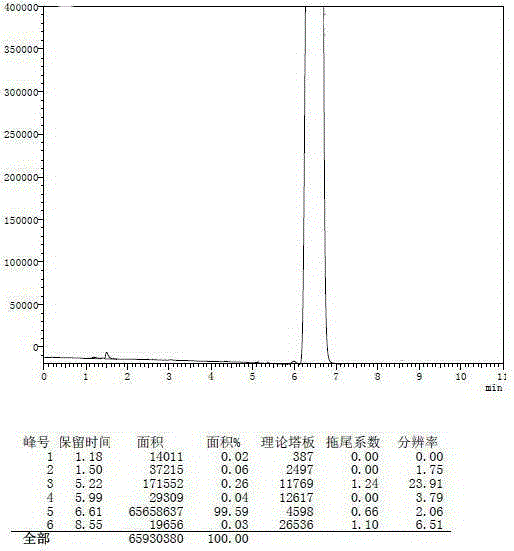
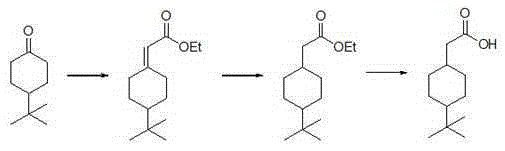
Synthetic method for 4-tertiary butyl cyclohexaneacetic acid
A technology for the synthesis of tert-butylcyclohexanone and its method is applied in the field of synthesis of chemical drug intermediates, achieving the effects of high yield, low price and simple post-treatment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] The synthetic method of 4-tert-butylcyclohexylacetic acid, step is as follows:
[0026] Step 1, Synthesis of ethyl 4-tert-butylcyclohexaneylidene acetate
[0027] Suspend 14.74g (0.37mol) of sodium hydride (commercially available content 60%) in 500mL dry tetrahydrofuran, stir and cool the reaction system to 0°C with a cold trap, drop 76.6mL (0.386mol) of triethyl phosphoroacetate Add to the above reaction system and stir at 0°C for 15 minutes, dissolve 54g (0.35mol) of 4-tert-butylcyclohexanone in 100mL tetrahydrofuran, add dropwise to the above reaction system at 0°C, after the addition is complete The temperature of the reaction system was raised to room temperature, and the reaction was stirred at room temperature for another 2 hours, then the reaction was quenched with 500 mL of water, concentrated under reduced pressure to remove part of tetrahydrofuran, and then the aqueous phase was extracted with 300 mL×2 methyl tert-butyl ether, and the organic phase was Comb...
Embodiment 2
[0033] Embodiment 2: comparative example
[0034] 1) Synthesis of ethyl 4-tert-butylcyclohexaneylidene acetate (adjust the molar ratio of 4-tert-butylcyclohexanone to triethyl phosphoroacetate to 1:1.3)
[0035] Suspend 14.74g (0.37mol) of sodium hydride (commercially available content 60%) in 500mL dry tetrahydrofuran, stir and cool the reaction system to 0°C with a cold trap, drop 76.6mL (0.386mol) of triethyl phosphoroacetate Add it to the above reaction system and stir at 0°C for 15 minutes, dissolve 45.7g (0.296mol) of 4-tert-butylcyclohexanone in 100mL tetrahydrofuran, add it dropwise to the above reaction system at 0°C, and the addition is complete Afterwards, the temperature of the reaction system was raised to room temperature, and the reaction was stirred at room temperature for another 2 hours, then the reaction was quenched with 500ml of water, concentrated under reduced pressure to remove part of tetrahydrofuran, and then the aqueous phase was extracted with 300mL...
Embodiment 3
[0040] Embodiment 3: comparative example
[0041] Synthesis of ethyl 4-tert-butylcyclohexyl acetate (increase the hydrogen pressure to 3MPa during the hydrogenation reduction reaction)
[0042]Place 50 g (0.22 mol) of ethyl 4-tert-butylcyclohexaneylidene acetate and 500 mL of methanol in a 1L high-pressure hydrogenation reactor, add 2.9 g of Raney nickel, replace the hydrogenation tank with hydrogen for three times, and pressurize with hydrogen after removing the air To 3MPa, heat the system to 55°C to carry out the hydrogenation reduction reaction. The reaction is considered to be over when the pressure no longer drops. The whole reaction takes 3.5 hours (when the hydrogen pressure is lower than 2MPa during the reaction, replenish hydrogen in time to keep the reaction Hydrogen pressure is 3MPa). Then the pressure was released, the reaction system was filtered to remove Raney nickel, and the filtrate was concentrated to obtain 48.1 g of oily product, namely ethyl 4-tert-butyl...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com