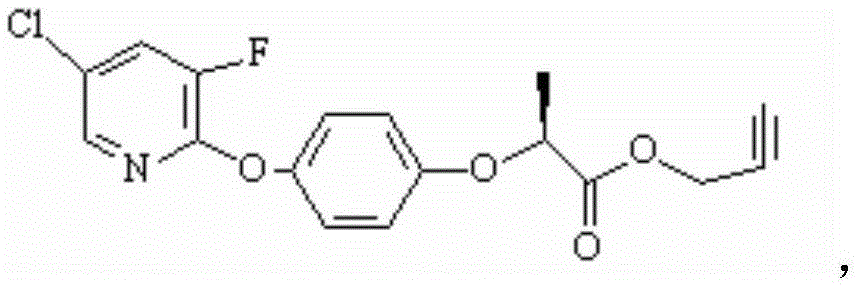
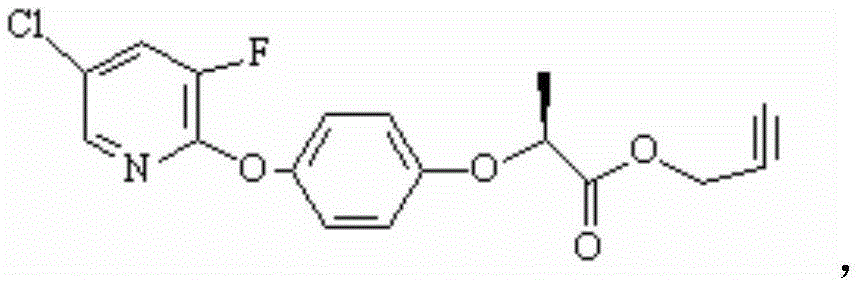
Preparation method of clodinafop propargyl
A technology of clodinafop-propargyl and clodinafop-propargyl acid is applied in the field of preparation of clodinafop-propargyl, which can solve the problems of affecting product quality, low yield, and difficulty in recovering solvent for product purification reaction, and achieves environmental friendliness in the preparation process, content and optical properties. The effect of high purity and good industrial application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0038] The preparation method of example 1 clodinafop-propargyl
[0039] The first step: preparation of clodinafop-propargyl acid
[0040] The preparation reaction formula of clodinafop-propargyl acid is as follows:
[0041]
[0042] The specific preparation method is as follows:
[0043] Add the following materials in the reactor: R-2-(p-hydroxyphenoxy) propionic acid (99.5% optical purity, 99% content) 183Kg (1kmol), water 75L, acetonitrile 600L and potassium hydroxide 112Kg (1.99kmol). The material was reacted at 40° C. for 2 hours with stirring. Add 171Kg (1.15Kmol) of 5-chloro-2,3-difluoropyridine (content: 96%), heat up to 75-85°C for reflux reaction for 6 hours, follow the reaction by HPLC, when the raw material R-2-(p-hydroxyphenoxy When the propionic acid is less than 1%, the reaction is stopped, and acetonitrile and a small amount of unreacted 5-chloro-2,3-dichloropyridine are recovered under reduced pressure. Add 400L of water to the reaction kettle and stir ...
Embodiment 2
[0049] The first step: preparation of clodinafop-propargyl acid
[0050] 20L of water, 40L of dimethyl sulfoxide, and 9.5Kg (237mol) of sodium hydroxide were added to the reaction kettle, and 18Kg of R-2-(p-hydroxyphenoxy)propionic acid (99.5% optical purity, 99% content) was added under stirring. (99mol), heated to 45°C for 1 hour under stirring. Add 17Kg (113.7mol) of 5-chloro-2,3-difluoropyridine, raise the temperature to 95°C for 6 hours, follow the reaction by HPLC, when the raw material R-2-(p-hydroxyphenoxy)propionic acid is less than 1% , stop responding. The solvent was recovered under reduced pressure, 40 L of water was added to the residue and stirred to dissolve, and the pH was adjusted to 3 with 15% hydrochloric acid, the solid was precipitated under stirring, filtered, and dried to obtain 30 Kg of clodinafop-argyl acid. Yield 97.1%, content 99.1%, optical purity 99.2%.
[0051] The second step: the preparation of clodinafop-propargyl
[0052] Add 30Kg (96mol)...
Embodiment 3
[0054]The first step: preparation of clodinafop-propargyl acid
[0055] 80L of water, 750L of acetonitrile, and 53Kg (946mol) of potassium hydroxide were added to the reaction kettle, and 90Kg (494.5mol) of R-2-(p-hydroxyphenoxy) propionic acid (99.5% optical purity, 99% content) was added under stirring. , stirred at room temperature for 3 hours. Add 85Kg (568.5mol) of 5-chloro-2,3-difluoropyridine, heat up to 75-85°C for reflux reaction, follow the reaction by HPLC, when the raw material R-2-(p-hydroxyphenoxy)propionic acid is less than 1% , stop the reaction. The solvent was recovered under reduced pressure, 200L of water was added to the residue and stirred to dissolve, and the pH was adjusted to 3 with 15% hydrochloric acid, the solid was precipitated under stirring, filtered, and dried to obtain 148Kg of clodinafop-propargyl acid. Yield 94.8%, content 99.2%, optical purity 98.2%.
[0056] The second step: the preparation of clodinafop-propargyl
[0057] Add 130Kg (41...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com