Ropivacaine freeze-drying agent capable of forming micelle
A technology of freeze-dried preparations and ropivacaine, which is applied in the field of medicine, can solve problems such as the inability to achieve sustained release, and achieve the effect of preventing organic acid salts and hydrolysis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Embodiment 1: Preparation of polyethylene glycol monomethyl ether-polylactic acid block copolymer
[0020] Weigh polyethylene glycol monomethyl ether 2000 and lactide with a mass ratio of 50:50 and place them in a three-neck flask, stir under nitrogen protection, and heat the reaction temperature to 130°C to make polyethylene glycol monomethyl ether In 2000, the lactide was melted, and after stirring and mixing, 0.2% of the total mass of stannous octoate was added, heated to 140°C under nitrogen protection, and stirred for 6 hours to obtain a colorless, clear and transparent viscous liquid; add dichloromethane to dissolve, and Precipitate with ice-cold anhydrous ether, filter the precipitate, and dry it under vacuum at room temperature for 24 hours. A polyethylene glycol monomethyl ether-polylactic acid block copolymer with an average relative molecular weight of 4000 was obtained, and the block ratio was 50:50.
[0021] According to the above method, weigh 90:10, 60:4...
Embodiment 2
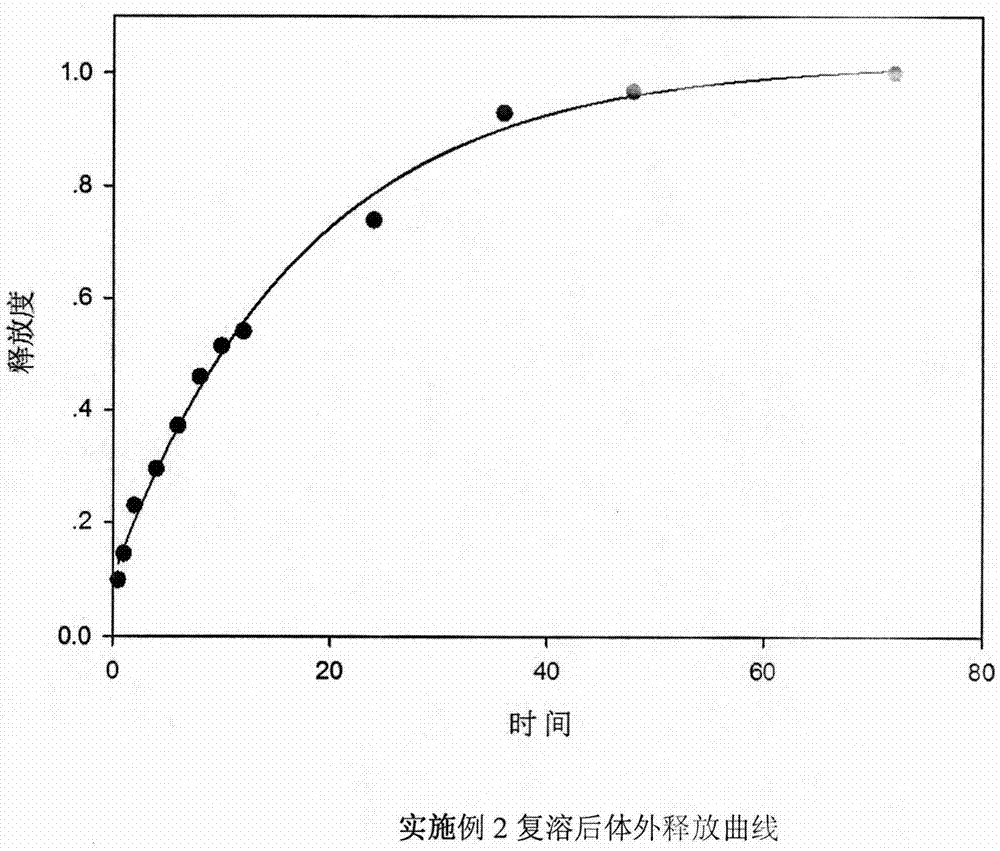
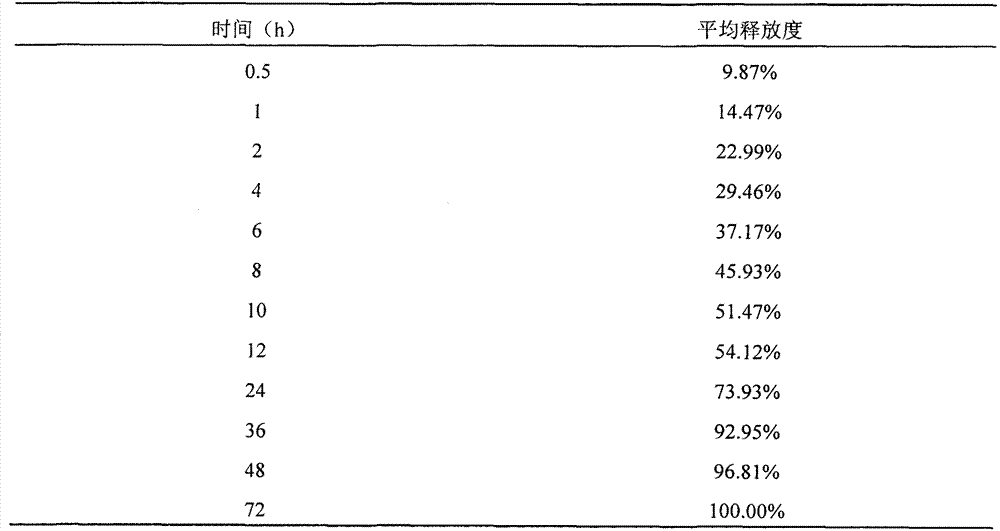
[0022] Embodiment 2: the preparation of ropivacaine freeze-dried preparation
[0023] Dissolve 200 mg of polyethylene glycol monomethyl ether-polylactic acid block copolymer (block ratio 50:50) in 2-tert-butanol, add 20 mg of ropivacaine to obtain a clear solution, filter it and add it to a vial , freeze-dried in a lyophilizer after half-capping, and the finished product is obtained by capping and capping.
Embodiment 3
[0024] Embodiment 3: the preparation of ropivacaine freeze-dried preparation
[0025] Dissolve 200 mg of polyethylene glycol monomethyl ether-polylactic acid block copolymer (block ratio 40:60) in 2 mL of tert-butanol and 0.05 mL of water, add 20 mg of ropivacaine to obtain a clear solution, filter and add In the vials, freeze-dry in a freeze dryer after half-capping, and obtain the finished product by capping and rolling the cap.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com