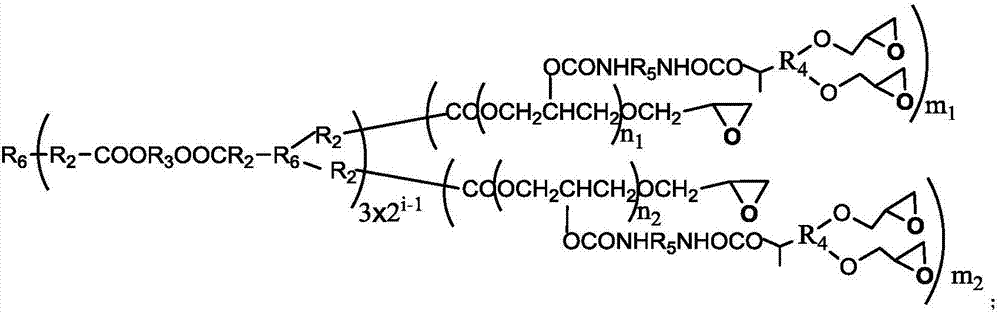
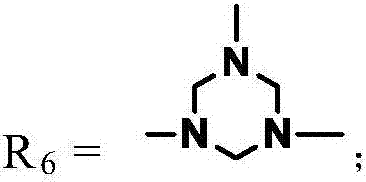
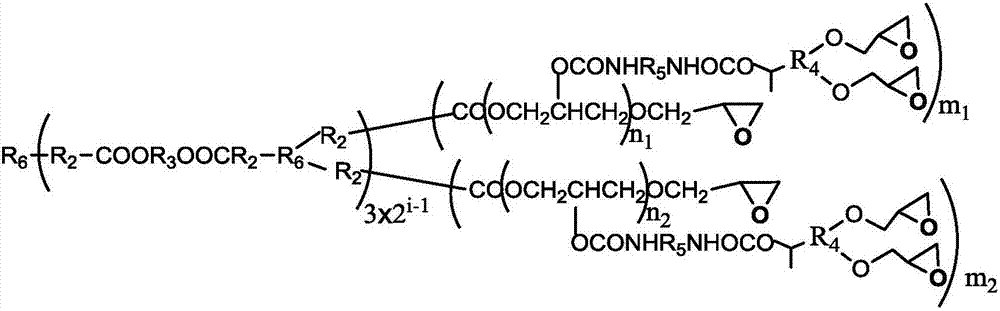
A kind of degradable self-crosslinking hyperbranched epoxy resin and preparation method thereof
An epoxy resin and self-crosslinking technology, applied in the field of degradable self-crosslinking hyperbranched epoxy resin and its preparation, can solve the problem that chemical bonds are difficult to degrade, recycling and reuse, serious environmental pollution, and restricting the sustainable development of epoxy resins. It can improve the storage stability, simplify the preparation process, and reduce the viscosity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] Preparation of compound B3:
[0037] The reaction principle of the preparation reference (Science 2014,344, (6185), 732-735) of cyclotriazine compound B3, the specific process steps are as follows: 0.1mol p-aminobenzoic acid (R 1 =-H, R 2 =-C 6 h 4 -), a concentration of 37wt% formaldehyde (containing 0.40mol of formaldehyde) was added in a three-necked flask with a condenser, a thermometer and a stirrer, and the reaction was stirred and reacted at a temperature of about 20° C. for 6 hours, and the reaction was stopped. The organic solvent was removed by rotary evaporation at about 60° C. under a vacuum of 2-3 mmHg to obtain a solid powder with a yield of about 75%. The triazine compound was designated as B3-006. All the other B3 compounds can be obtained with a similar method, and the productive rate is between 60-85%, and the corresponding compound is denoted as B3-112 (R 1 =-CH 3 , R 2 =-C 6 h 4 OC 6 h 4 -), B3-206(R 1 =-C 2 h 5 , R 2 =-C 6 h 4 -), B3...
Embodiment 1
[0043] (a) 0.133mol cyclic triazine compound B3-006, 0.1mol ethylene glycol, 0.1mol xylene, zinc acetate (the quality of zinc acetate is 0.5% of the total mass of B3-006 and ethylene glycol) are mixed uniformly, at 180 ℃ for stirring reaction for 6 hours, and then vacuumize the xylene at 110 ℃ to obtain a carboxyl-terminated hyperbranched polymer (TDHBP-006, containing 6 mol carboxyl groups per mole of TDHBP-006), with a number average molecular weight of about 1800 g / mol.
[0044] (b) Add 0.01mol TDHBP-006, 0.3mol epichlorohydrin, and 0.0015mol boron trifluoride etherate complex into a three-necked flask, stir and react at about 120°C for 6 hours, and then vacuum out excess epichlorohydrin Propane, add 0.45mol tetrahydrofuran and 0.3mol sodium hydroxide, then stir and react at -5~0°C for 10 hours, stop the reaction, separate layers, wash with water until neutral, and distill off the organic solvent to obtain a hyperbranched ring containing hydroxyl Oxygen resin (TDHEP-006), t...
Embodiment 2
[0047] (a) Mix 0.10mol cyclic triazine compound B3-112, 0.09mol butanediol, 0.27mol xylene, p-toluenesulfonic acid (the quality of p-toluenesulfonic acid is 0.1% of the total mass of B3-112 and butanediol) Uniform, stirred and reacted at 140°C for 10h, then vacuumed out the xylene at 120°C to obtain a methyl-terminated hyperbranched polymer (TDHBP-112a, containing 12mol ester groups per mole of TDHBP-112a), with a number average molecular weight of about 7500g / mol.
[0048] (b) Add 0.005mol TDHBP-112a, 0.6mol epichlorohydrin, and 0.006mol tetrabutylammonium bromide into a three-necked flask, stir and react at about 110°C for 8 hours, and then vacuum out excess epichlorohydrin, Add 0.30mol ethyl acetate and 0.06mol sodium hydroxide, then stir and react at 0-5°C for 8 hours, stop the reaction, separate layers, wash with water until neutral, and distill off the organic solvent to obtain a hydroxyl-containing hyperbranched epoxy Resin (TDHEP-112a), the number average molecular we...
PUM
| Property | Measurement | Unit |
|---|---|---|
| epoxy value | aaaaa | aaaaa |
| epoxy value | aaaaa | aaaaa |
| epoxy value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com