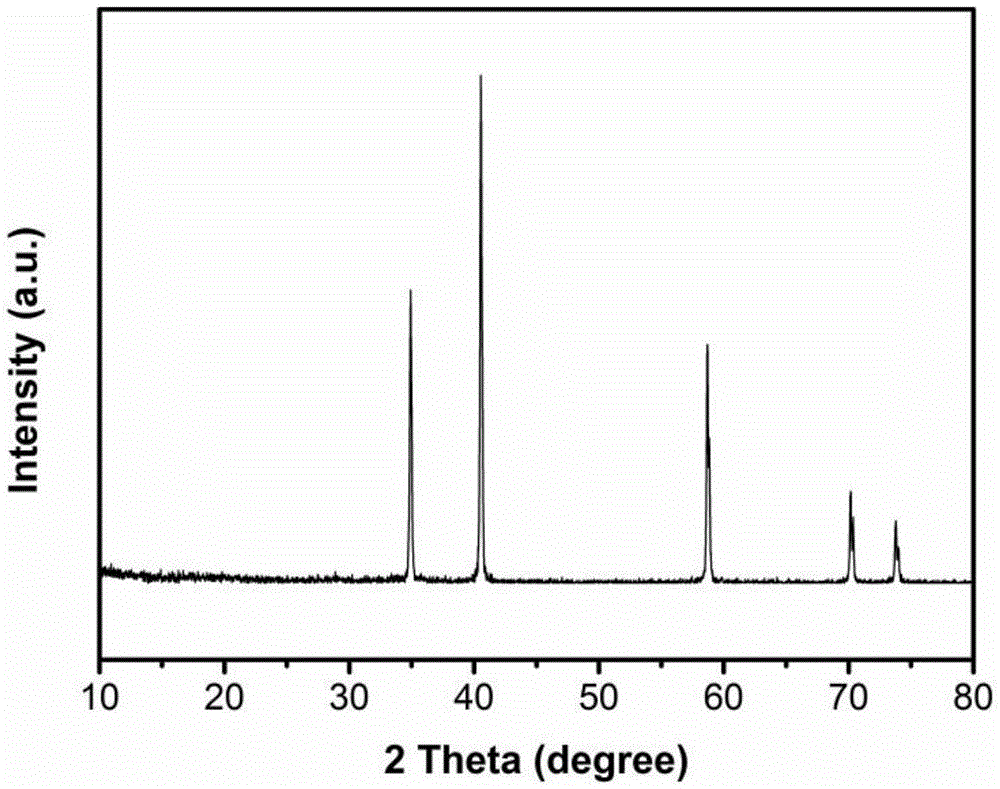
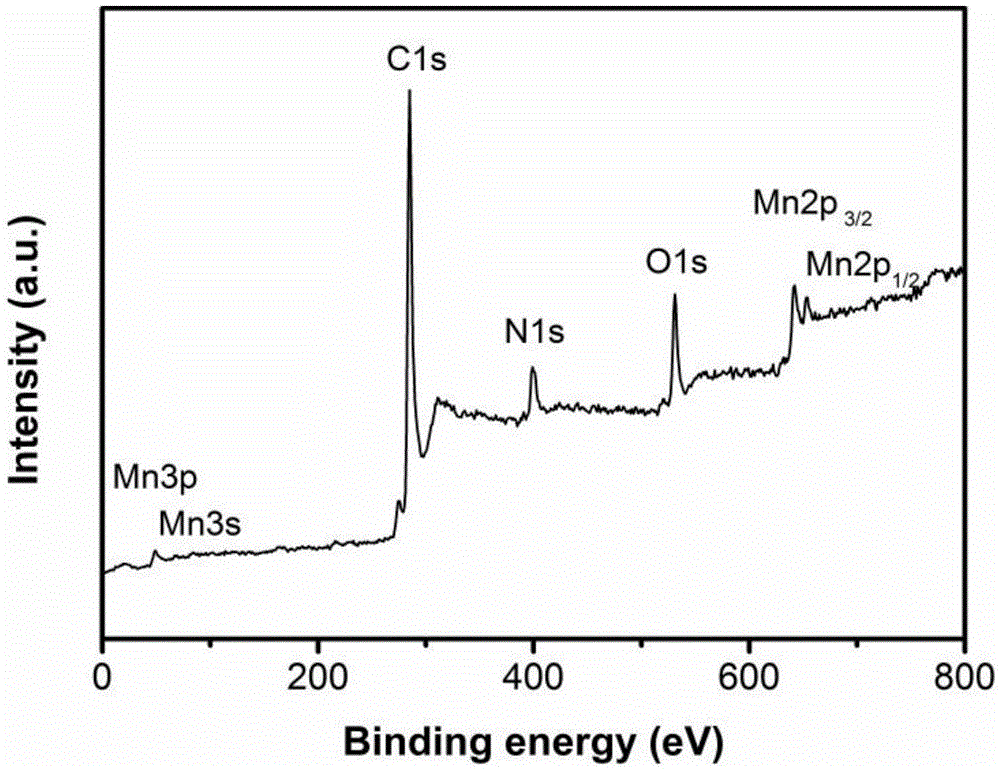
Nitrogen-doped carbon-coated manganese monoxide composite material with one-dimensional porous core-shell structure and preparation method of nitrogen-doped carbon-coated manganese monoxide composite material
A manganese monoxide, nitrogen-doped carbon technology, applied in structural parts, nanotechnology for materials and surface science, electrochemical generators, etc., can solve the problem of manganese monoxide low electronic conductivity, low reversible capacity, rate Poor performance and other problems, to achieve the effect of suppressing the problem of rapid capacity decay, high theoretical specific capacity, and improving rate performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] 1) Weigh 0.52g of manganese dioxide (MnO 2 ) nanorods, 0.46g ammonium persulfate ((NH 4 ) 2 S 2 o 8 ) into 30mL deionized water, ultrasonically treated with an ultrasonic cleaner for 30 minutes, then stirred in an ice bath for 15 minutes; manganese dioxide nanorods can be prepared by the following hydrothermal method: Weigh 1.35g manganese sulfate monohydrate (MnSO 4 ·H 2 O) and 1.83g ammonium persulfate ((NH 4 ) 2 S 2 o 8 ) was dissolved in 70mL of water, stirred for 30 minutes, transferred to a 100mL polytetrafluoroethylene reactor, hydrothermally reacted at 140°C for 12 hours, centrifuged and washed with clean water for 3 times, dried and set aside.
[0032]2) Mix 90 μL of aniline monomer (the molar ratio of aniline to ammonium persulfate is 1:2), 0.15 g of oxalic acid (H 2 C 2 o 4 ) into 24 mL of deionized water, and stirred in an ice bath for 15 minutes;
[0033] 3) After the mixed solution prepared in step 1) stops stirring, quickly add the solution of...
Embodiment 2
[0038] 1) Weigh 0.26g of manganese dioxide (MnO 2 ) nanorods, 0.46g ammonium persulfate ((NH 4 ) 2 S 2 o 8 ) into 30 mL of deionized water, ultrasonically treated with an ultrasonic cleaner for 5 minutes, and then stirred in an ice bath for 10 minutes;
[0039] 2) Mix 180 μL of aniline monomer (the molar ratio of aniline to ammonium persulfate is 1:1), 1.2 g of phytic acid (C 6 h 18 o 24 P 6 ) into 24 mL of deionized water, and stirred in an ice bath for 10 minutes;
[0040] 3) After the mixed solution prepared in step 1) stops stirring, quickly add the solution of step 2) into it, move it into the freezer at 0-10°C and let it stand for 0.5 hours, after washing with water for 3 times, dry it at 60°C to obtain the core-shell structure Manganese dioxide nanorods coated with polyaniline;
[0041] 4) Place the polyaniline-coated manganese dioxide nanorods in a tube furnace filled with argon, heat at 500 °C for 2 hours at a heating rate of 1 °C / min, and wait for the tube f...
Embodiment 3
[0043] 1) Weigh 0.52g of manganese dioxide (MnO 2 ) nanorods, 80 μL hydrogen peroxide ((H 2 o 2 ) into 30 mL of deionized water, ultrasonically treated with an ultrasonic cleaner for 30 minutes, then stirred in an ice bath for 15 minutes;
[0044] 2) Mix 60 μL of aniline monomer (the molar ratio of aniline to hydrogen peroxide is 1:4), 0.15 g of oxalic acid (H 2 C 2 o 4 ) into 24 mL of deionized water, and stirred in an ice bath for 15 minutes;
[0045] 3) After the mixed solution prepared in step 1) stops stirring, quickly add the solution of step 2) into it, move it into the freezer at 0-10°C and let it stand for 6 hours, and after washing with water for 3 times, dry it at 90°C to obtain the core-shell structure Manganese dioxide nanorods coated with polyaniline;
[0046] 4) Place polyaniline-coated manganese dioxide nanorods in a tube furnace filled with nitrogen, heat at 800 °C for 2 hours at a heating rate of 5 °C / min, and wait for the tube furnace to cool naturally...
PUM
| Property | Measurement | Unit |
|---|---|---|
| length | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com