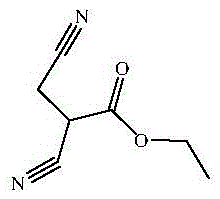
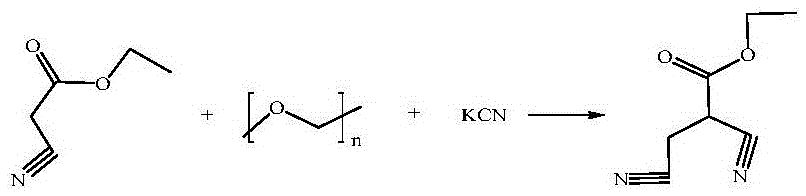Preparing method of 2,3-dicyanopropionate
A technology of ethyl dicyanopropionate and ethyl cyanopropionate is applied in the field of preparation of 2,3-ethyl dicyanopropionate, an intermediate of phenylpyrazole pesticides, and can solve the problem of improper operation , safety accidents, high requirements for operation and safety and environmental protection, to achieve the effect of low production cost, good product quality, and low processing difficulty
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
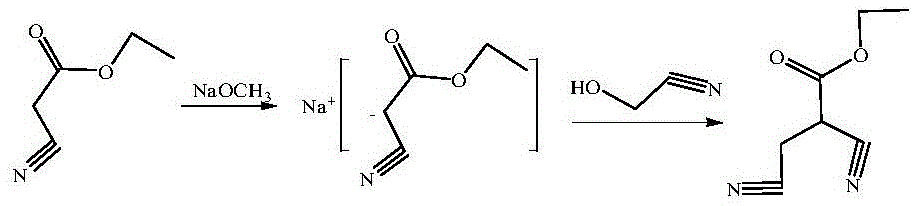
[0028] Put 168.2g (1.05mol) of diethyl malonate and 168.2g of methanol into the reaction flask for miscibility, drop in 272.3g (mass fraction 25%, 1.26mol) of methanol solution of sodium methoxide within 3 hours below 10°C, and drop it Cool to 0°C, slowly add 57.1g (1mol) of hydroxyacetonitrile dropwise within 1h, keep the temperature at 0°C for 1h after dropping; adjust the pH value of the reaction solution to 5 with saturated hydrochloric acid below 10°C, filter, and remove the solvent in the filtrate under reduced pressure Finally, the concentrated material was obtained, and 336.4g of dichloroethane was used to dissolve the concentrated material and soak the filter cake. The soaking liquid was combined into the concentrated material solution, filtered, and the filtrate was precipitated to obtain 173g of yellow liquid intermediate Iα-cyanomethyl- Diethyl malonate, content 95.2%, yield 82.7% (based on hydroxyacetonitrile).
[0029] Put 173g (0.83mol) of intermediate I and 138...
Embodiment 2
[0031] Put 168.2g (1.05mol) of diethyl malonate and 168.2g of methanol into the reaction flask for miscibility, drop in 272.3g (mass fraction 25%, 1.26mol) of methanol solution of sodium methoxide within 3 hours below 10°C, and drop it Cool to 0°C, slowly add 57.1g (1mol) of hydroxyacetonitrile dropwise within 1h, keep the temperature at 0°C for 1h after dropping; adjust the pH value of the reaction solution to 5 with saturated hydrochloric acid below 10°C, filter, and remove the solvent in the filtrate under reduced pressure Finally, the concentrated material was obtained, and 336.4g of dichloroethane was used to dissolve the concentrated material and soak the filter cake, and the soaking liquid was combined into the concentrated material solution, filtered, and the filtrate was precipitated to obtain 172.1g of yellow liquid intermediate Iα-cyanomethyl -diethyl malonate, content 95.3%, yield 82.3% (calculated as hydroxyacetonitrile).
[0032]Put 172g (0.82mol) of intermediate...
Embodiment 3
[0034] Put 176.2g (1.1mol) of diethyl malonate and 176.2g of methanol into the reaction flask for miscibility, drop in 285.2g (mass fraction 25%, 1.32mol) of methanol solution of sodium methoxide within 3 hours below 10°C, and drop it Cool to 0°C, slowly add 57.1g (1mol) of hydroxyacetonitrile dropwise within 1h, keep the temperature at 0°C for 1h after dropping; adjust the pH value of the reaction solution to 5 with saturated hydrochloric acid below 10°C, filter, and remove the solvent in the filtrate under reduced pressure Finally, the concentrated material was obtained, and 352.4g of dichloroethane was used to dissolve the concentrated material and soak the filter cake, and the soaking liquid was combined into the concentrated material solution, filtered, and the filtrate was precipitated to obtain 171.3g of yellow liquid intermediate Iα-cyanomethyl - Diethyl malonate, content 95.5%, yield 82.1% (calculated as hydroxyacetonitrile).
[0035] Put 171.3g (0.82mol) of intermedi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com