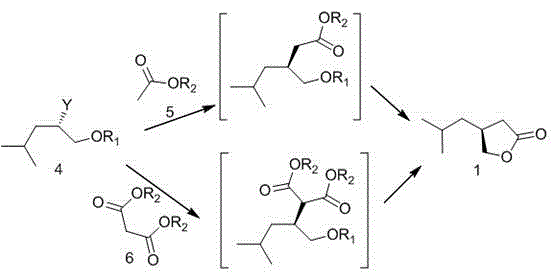
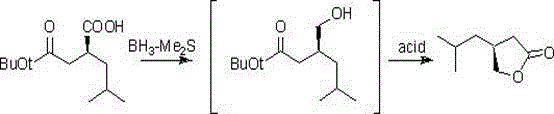
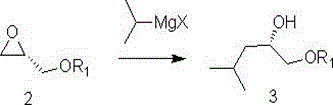Preparation method of pregabalin chiral intermediate
A chiral intermediate, pregabalin technology, applied in the field of organic synthesis, can solve problems such as difficulty in obtaining qualified optically pure products, easy generation of chiral isomers, etc., achieving a short route, easy to scale up production, and high overall yield Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Synthesis of (S)-1-tert-butoxy-4-methyl-2-pentanol (3a)
[0035]
[0036] Under nitrogen protection, a THF solution of isopropylmagnesium bromide (220ml, 1mol / L, 0.22mol) was added to a 1L three-necked flask, cooled to -5°C under stirring, and then a THF solution of compound 2a (26.04g dissolved in 40ml THF). After the dropwise addition, keep the temperature between -10°C and 0°C for 4 hours. After the reaction was completed, 200ml of water was added dropwise to the reaction system, and HOAc was used to adjust the pH to between 6-8. EA extraction (100mlx3), the combined organic phases were dried over anhydrous sodium sulfate, and concentrated under reduced pressure to remove the solvent to obtain 32.21 g of compound 3a as a colorless oily liquid with a yield of 92.4%. 1 HNMR (400MHz, CDCl 3 )δ3.72(dd,1H),3.59-2.64(m,1H),3.42(dd,1H),1.92(t,2H,),1.62-1.66(m,1H),1.23(S,9H), 0.87(d,6H).
Embodiment 2
[0038] Synthesis of (S)-1-tert-butoxy-4-methyl-2-pentanol (3a)
[0039]
[0040] Under nitrogen protection, a THF solution of isopropylmagnesium chloride (220ml, 1mol / L, 0.22mol) was added to a 1L three-necked flask, stirred and cooled to -5°C, and then the THF solution of compound 2a (26.04g dissolved in 40mlTHF). After the dropwise addition, keep the temperature between -10°C and 0°C for 6 hours. After the reaction was completed, 200ml of water was added dropwise to the reaction system, and HOAc was used to adjust the pH to between 6-8. EA extraction (100mlx3), the combined organic phases were dried over anhydrous sodium sulfate, and concentrated under reduced pressure to remove the solvent to obtain 29.88g of a colorless oily liquid of compound 3a, with a yield of 85.7%.
Embodiment 3
[0042] Synthesis of (S)-1-tert-butoxy-4-methyl-2-pentyl trifluoromethanesulfonate (4a)
[0043]
[0044] Add 3a (34.85g, 0.20mol) and dichloromethane (340ml) into a 1L three-necked flask, stir and cool to -10°C under nitrogen protection, then add triethylamine (24.29g, 0.24mol). Keep warm and slowly add trifluoroacetic anhydride (59.25 g, 0.21 mol) dropwise. After the dropwise addition, keep the temperature at -10°C for 1 hour. Add 1.0M sodium bicarbonate solution (200ml) to the reaction system, stir for 10min, then let stand, separate the organic layer, and extract the aqueous layer with dichloromethane (150ml); the combined organic layer is dried over anhydrous sodium sulfate, in The solvent was removed under reduced pressure at less than 35°C to obtain compound 4a as a light yellow oily liquid, which was directly used in the next reaction.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com