Maleate cis-trans isomerase mutant, and coding gene and application thereof
A technology of cis-trans isomerase and maleic acid, applied in the field of maleate cis-trans isomerase mutants, can solve the problems of many by-products, environmental pollution, and high cost of chemical synthesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] The acquisition of embodiment 1 maleate cis-trans isomerase gene
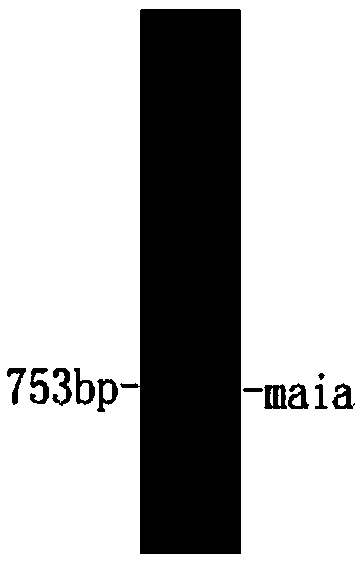
[0041] Using 1 μg of Serratia marcescens (Serratiamarcescens) genomic DNA as a PCR reaction template, the forward primer MaiA-F was designed according to the sequence of SEQ ID NO. CCCAAGCTTATAAGCGCCGGACAG-3′, where the parts in italics are the enzyme cutting sites EcoRI and HindIII respectively. The PCR reaction was carried out in a total volume of 50 μl. The reaction conditions were 30 cycles of denaturation at 94°C for 5 min, denaturation at 94°C for 50 s, annealing at 58°C for 1 min, and extension at 72°C for 2 min. Take 3 μl of PCR amplification products for agarose gel electrophoresis verification, the results are as follows figure 1 shown. Take 100 μl of the PCR product for agarose gel electrophoresis, and recover the target fragment according to the steps of the gel recovery kit.
Embodiment 2
[0042] Embodiment 2 Construction of wild-type maleic acid cis-trans isomerase gene expression vector
[0043] In Example 1, the PCR product was digested with restriction endonucleases EcoRI and HindIII, and then ligated with the pET-24a (+) plasmid (purchased from Novagen) digested with EcoRI and HindIII endonucleases. The constructed The vector is called pET-MaiA, and then the pET-MaiA mixture is used to transform Escherichia coli DH5-α (purchased from promega company), and carry out sequence determination to extract the plasmid of the transformed cell library, and transform Escherichia coli BL21 (DE3) strain ( purchased from promega company).
Embodiment 3
[0044] Example 3 Error-prone PCR amplification of Serratia marcescens maleate cis-trans isomerase gene
[0045]Using the property that TaqDNA polymerase does not have 3'-5' proofreading function, the frequency of random mutations can be controlled at high magnesium ion concentration (5-9mmol / L) and different concentrations of dNTP (1.5-3.5mmol / L) , introduce random mutations into the target gene, and construct a mutation library. The optimal mutation rate in the experiment is about 0.6%.
[0046] Error-prone PCR reaction system (100μl):
[0047]
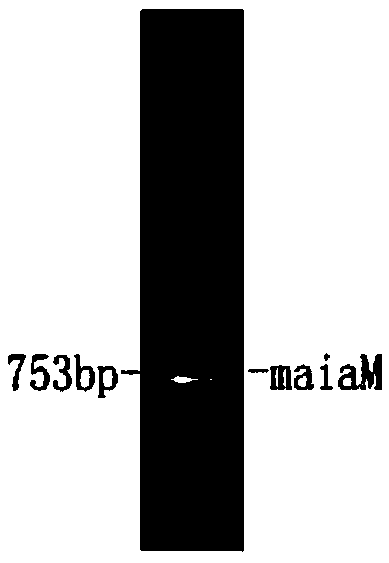
[0048] The PCR program was: pre-denaturation at 96°C for 4 minutes, denaturation at 94°C for 1 minute, annealing at 56°C for 1 minute, extension at 75°C for 2 minutes, 45 cycles of reaction, and finally extension at 75°C for 15 minutes. Take 5 μ l of the product and check it with agarose gel electrophoresis, the result is as follows: figure 2 shown. The PCR product was recovered by gel recovery method and stored at -20°C for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com