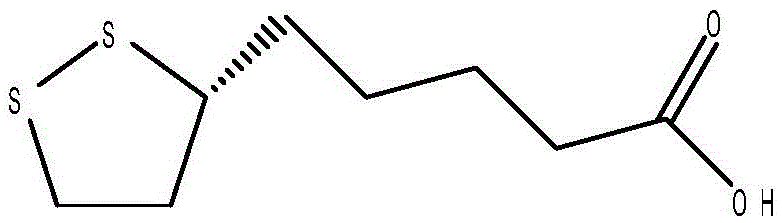
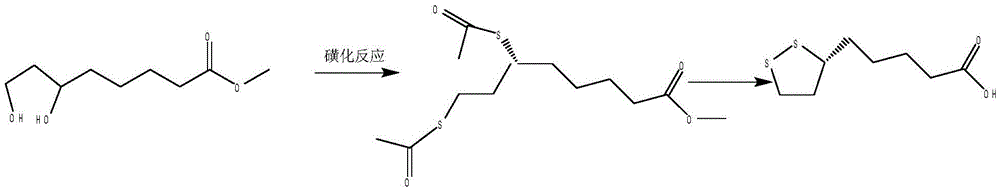
Preparation method of (R)-(+)-lipoic acid
A technology of lipoic acid and ethyl dichlorooctanoate, which is applied in the field of preparation of R-lipoic acid, can solve the problems of inability to meet industrialized scale-up production, does not conform to green technology, and high waste discharge, and can meet the requirements of industrial scale-up production and process steps. The effect of reducing and reducing waste discharge
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
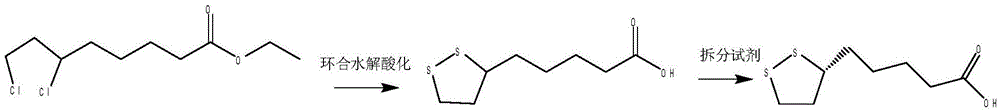
[0035] A) Preparation of cyclization solution: put (S)-6,8-dichlorooctanoic acid ethyl ester and sulfur into a reaction vessel equipped with a stirring device, heat up to 70°C, and add dropwise an 8% sodium sulfide aqueous solution Carry out the cyclization reaction, the time of the cyclization reaction is 5 hours, the molar ratio of (S)-6,8-dichlorooctanoic acid ethyl ester, sulfur and sodium sulfide aqueous solution is 1:1.7:1, keep the temperature at 70°C for 2h, After the cyclization reaction is completed, use an organic solvent, that is, extract with toluene, and concentrate to obtain a cyclization liquid;
[0036] B) Preparation of hydrolyzate: the cyclization solution obtained by step A) is put into a reaction vessel equipped with a stirring device and added with a mass percent concentration of 4% sodium hydroxide solution for hydrolysis reaction, and the cyclization solution and mass percent concentration are The molar ratio of the 4% sodium hydroxide solution is 1:1.5...
Embodiment 2
[0040] A) Preparation of cyclization solution: put (S)-6,8-dichlorooctanoic acid ethyl ester and sulfur into a reaction vessel equipped with a stirring device, heat up to 80°C, and add dropwise an aqueous sodium sulfide solution with a concentration of 20% by mass Carry out cyclization reaction, the time of cyclization reaction is 0.5 hours, the molar ratio of (S)-6,8-dichlorooctanoic acid ethyl ester, sulfur and sodium sulfide aqueous solution is 1:1.5:1, keep warm at 80°C for 1.5h , the cyclization reaction is completed, and the organic solvent is used to extract with ethyl acetate, and concentrated to obtain the cyclization liquid;
[0041] B) Preparation of hydrolyzate: the cyclization solution obtained by step A) is dropped into a reaction vessel equipped with a stirring device and added with a concentration of 3% potassium hydroxide for hydrolysis reaction, and the cyclization solution and mass percent concentration are The molar ratio of the 3% potassium hydroxide solut...
Embodiment 3
[0045] A) Preparation of cyclization solution: put (S)-6,8-dichlorooctanoic acid ethyl ester and sulfur into a reaction vessel equipped with a stirring device, heat up to 90°C, and add dropwise an aqueous sodium sulfide solution with a concentration of 12% by mass Carry out the cyclization reaction, the time of the cyclization reaction is 3 hours, the molar ratio of (S)-6,8-dichlorooctanoic acid ethyl ester, sulfur and sodium sulfide aqueous solution is 1:2:1.5, keep the temperature at 90°C for 1h, After the cyclization reaction is completed, use an organic solvent, that is, extract with cyclohexane, and concentrate to obtain a cyclization liquid;
[0046] B) Preparation of hydrolyzate: put the cyclization solution obtained by step A) into a reaction vessel equipped with a stirring device and add a sodium carbonate solution with a mass percent concentration of 8% to carry out the hydrolysis reaction, and the cyclization solution and mass percent concentration are 8% The molar ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Optical rotation | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com