Separation of hydrogen sulfide from natural gas
A technology of hydrogen sulfide and separation method, which is applied in the direction of separation method, dispersed particle separation, gas fuel, etc., and can solve the problem of selectivity reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
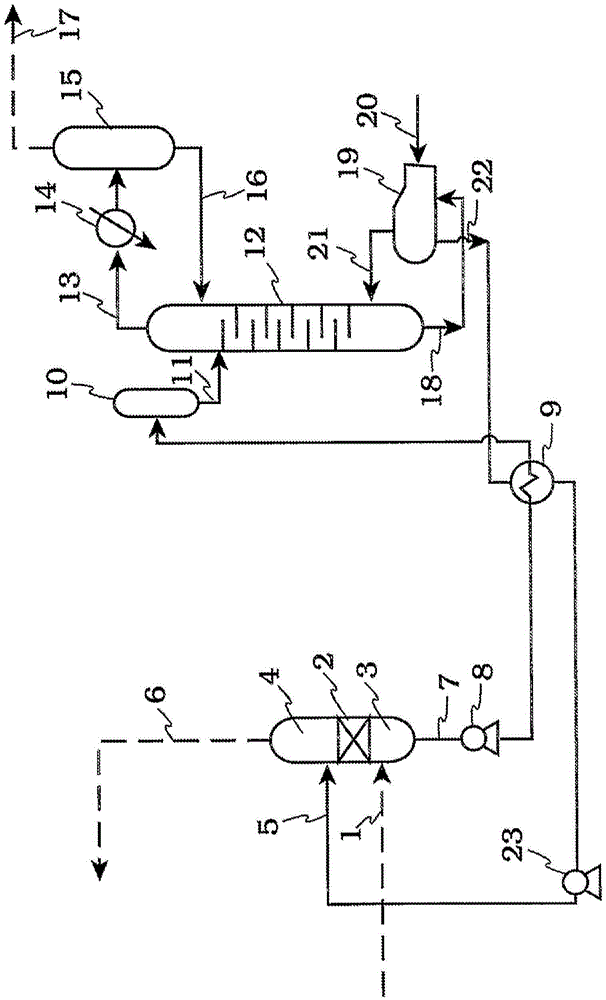
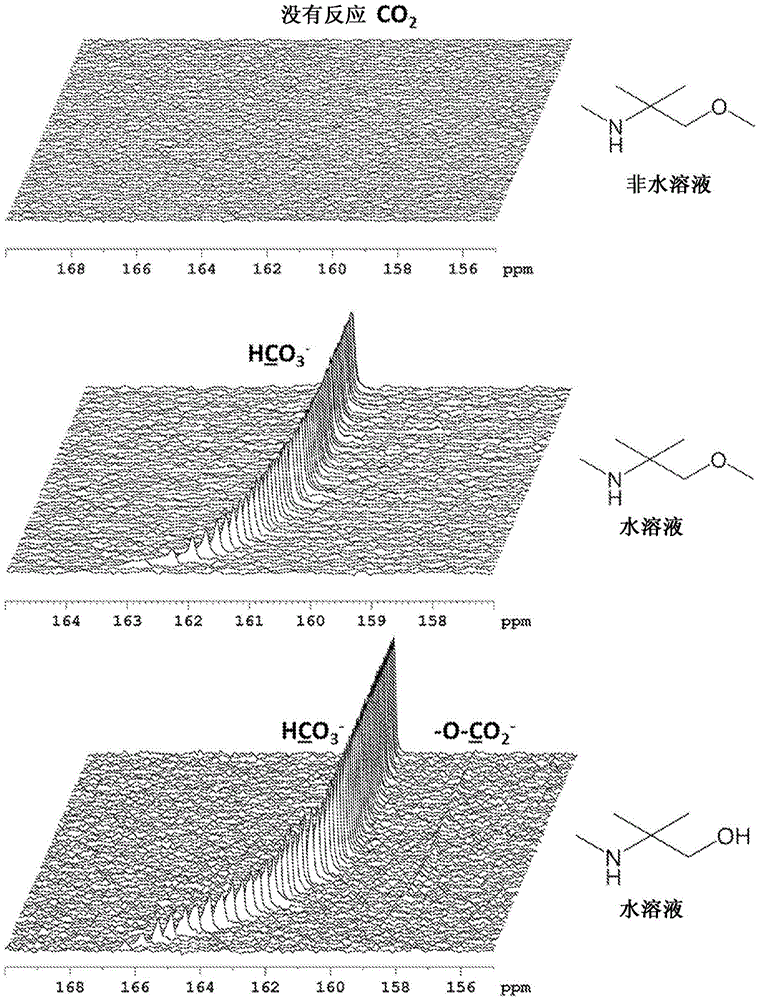
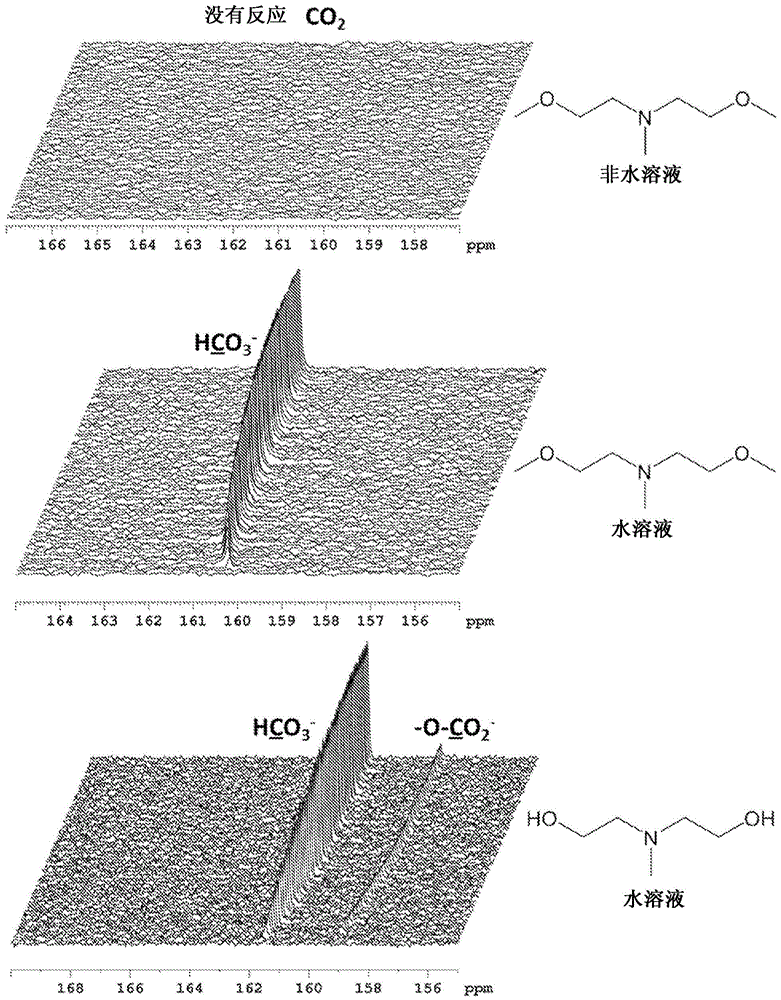
Image
Examples
Embodiment 1
[0140] The synthesis of embodiment 1,2-methoxyethyl-N-methyl-ethanolamine (MDEA-OMe)
[0141]
[0142]The secondary amine 2-N-methylaminoethanol (3.76g, 0.05mol), N,N-diisopropylethylamine (DIPEA) (6.46g, 0.075mol), 2-methoxyethyl bromide ( 7.30 g, 0.0525 mol) and 30 mL of acetonitrile were placed in a round bottom flask and stirred at room temperature under nitrogen. After completion of the reaction, (~6 h, monitored by HPLC) the reaction mixture was evaporated under reduced pressure in a rotary evaporator. The residue was dissolved in 50 mL of dichloromethane and washed with 50 mL of 50% aqueous sodium hydroxide. The aqueous layer was partially washed with 3 x 15 mL of dichloromethane. The collected organic fractions were dried over sodium sulfate, and then the solvent was removed under reduced pressure in a rotary evaporator at a low temperature of 0-5 °C to produce a crude product, which was finally purified by fractional vacuum distillation under sodium hydroxide. C...
Embodiment 2
[0144] Embodiment 2, two (2-methoxyethyl)-N-methylamine (MDEA-(OMe) 2 )Synthesis
[0145]
[0146] Bis(2-methoxyethyl)amine (35.45 g, 0.26 mol) was cooled to 0°C in a 2L round bottom flask containing a stirrer bar. After 88% aqueous formic acid (47 mL, 0.91 mol) was added dropwise, 37% aqueous formaldehyde (56 mL, 0.69 mol) was added. Rapid gas evolution started after controlled heating to 60°C. The reaction was allowed to proceed without further heating until gas evolution subsided (~6h) then heated to 80°C for 24h. The reaction mixture was cooled, acidified with 20% aqueous HCl, and partially extracted three times with 100 mL of diethyl ether. The aqueous layer was stirred in a salt ice bath and the pH was brought to 12 by the dropwise addition of 40% aqueous NaOH without letting the internal temperature exceed 25°C. After separation of the resulting amine / water layer, the aqueous layer was further partially extracted three times with 100 mL of diethyl ether. The com...
Embodiment 3
[0148] Example 3, 2-methyl-3-methoxy-2-propylamine (AP-OMe) and 2,2-dimethyl-3-methoxy-2-propylamine (AMP-OMe) synthetic general steps
[0149] This example demonstrates the synthesis of two alkoxypropylamine derivatives in a tertiary synthesis in which the initial propanolamine compound is first protected by p-methoxyphenyl protection (PMP protection) , to form a protected aminoalcohol, which is then methylated on the hydroxyl group, followed by removal of the protective PMP group to form the final methoxy-substituted amine.
[0150] Step 1 p-methoxyphenyl protection (PMP protection)
[0151]
[0152] A mixture of the selected aminoalcohol (1 equiv) and anisaldehyde (1.1 equiv) was heated at reflux in benzene during 24 hours while removing water azeotropically. The reaction was concentrated under reduced pressure. The desired product was recrystallized from hexane.
[0153] PMP-AP-OH was collected as white crystallites in 96% yield. 1 HNMR (300MHz, CDCl 3 )δ8.09(s, 1H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| pore size | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com