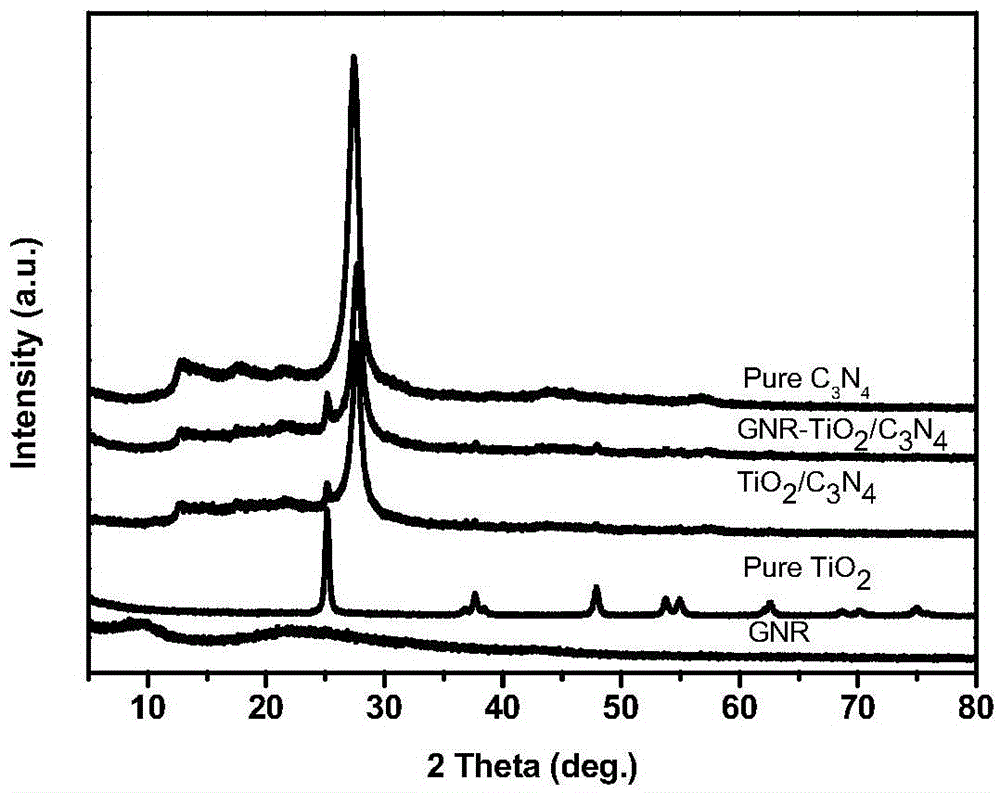
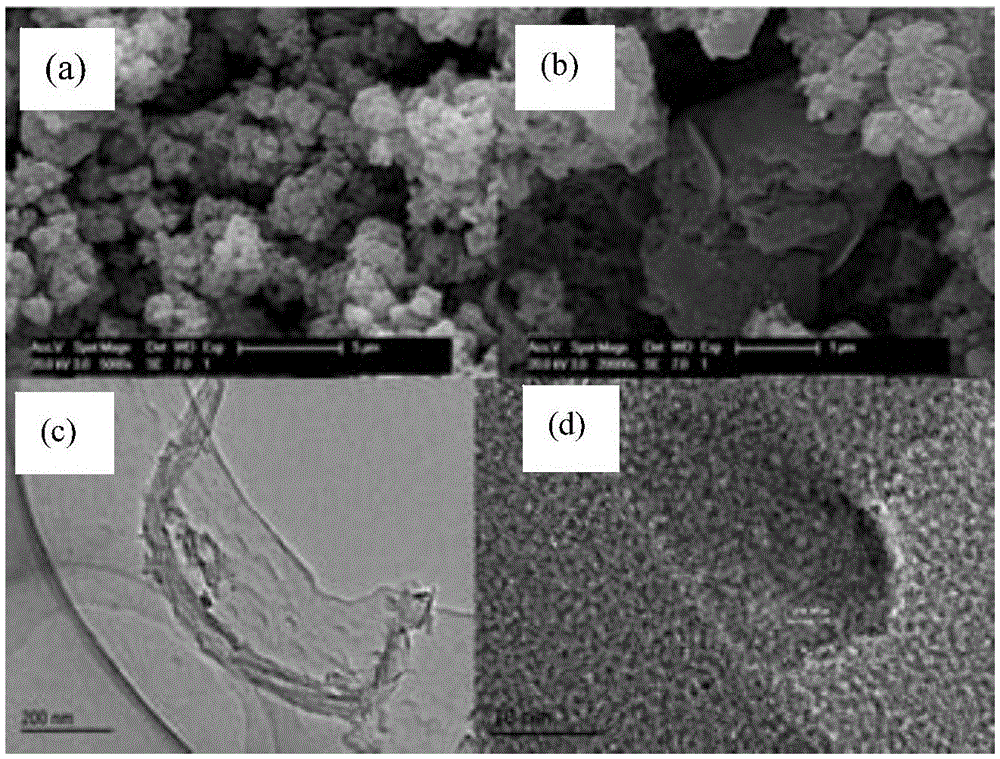
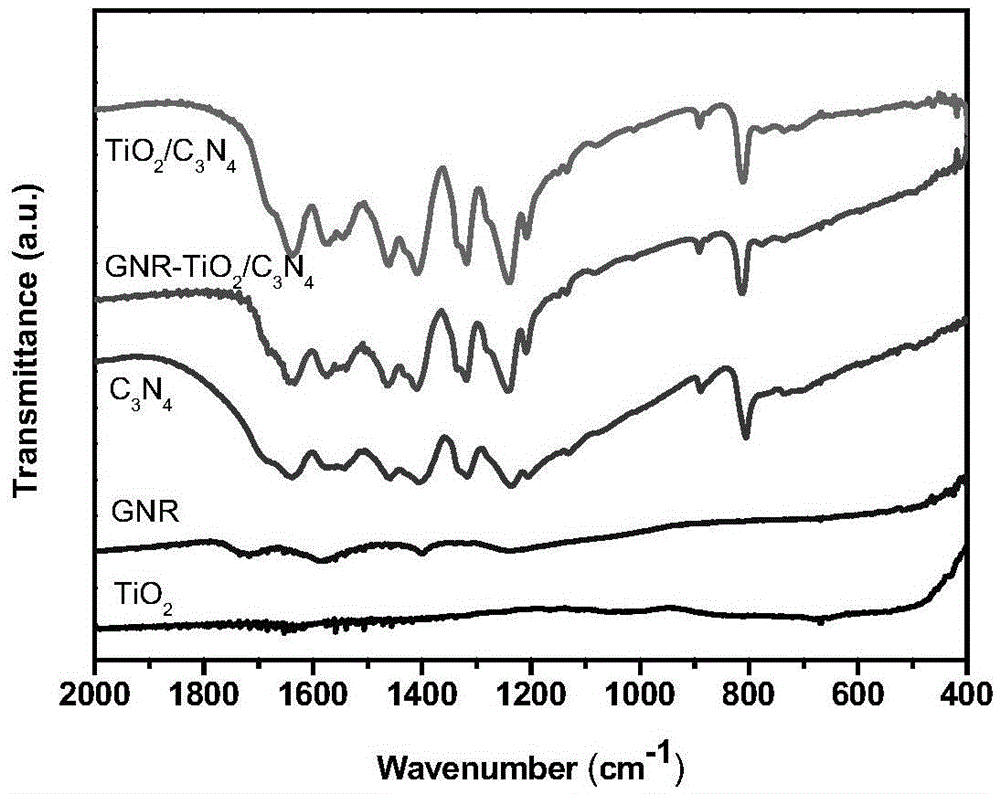
Preparation method of graphene nanobelt-loaded semi-conductive 3D photocatalytic material
A technology of graphene nanobelts and photocatalytic materials, which is applied in the field of preparation of three-dimensional photocatalytic materials, to achieve the effect of simple preparation process, improved activity and photocatalytic performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] Graphene nanoribbon loaded semiconductor material GNR-TiO 2 / C 3 N 4 , containing C, H, Ti, O and H elements.
[0028] The above-mentioned graphene nanobelt loaded semiconductor material specifically includes the following steps:
[0029] (1) Preparation of graphene nanobelts
[0030] Graphene nanoribbons (GNR) were prepared by the following method: weigh 1.5 g of multi-walled carbon nanotubes, add 150 mL of concentrated sulfuric acid, and stir for 1 h. Under ice-bath conditions, slowly add 9 g of potassium permanganate, and stir for 2 h at 25° C. Then in an oil bath at 75 °C for 80 min. Under ice bath conditions, add 25mL of distilled water, then pour the above solution into a large beaker filled with 300-400mL of distilled water, and then add 5mL of hydrogen peroxide. The product GNR was obtained by centrifugation.
[0031] (2) Preparation of graphene nanobelt-supported semiconductor materials carbon nitride and titanium dioxide
[0032] Weigh 0.3g of titanium d...
Embodiment 2
[0044] A three-dimensional photocatalytic material GNR-TiO supported by graphene nanoribbons 2 / Bi 2 WO 6 . Contains C, Ti, O, H, Bi, W elements.
[0045] (1) Preparation of graphene nanobelts
[0046] Graphene nanoribbons (GNR) were prepared by the following method: weigh 1.5 g of multi-walled carbon nanotubes, add 150 mL of concentrated sulfuric acid, and stir for 1 h. Under ice-bath conditions, slowly add 9 g of potassium permanganate, and stir for 2 h at 25° C. Then in an oil bath at 75 °C for 80 min. Under ice bath conditions, add 25mL of distilled water, then pour the above solution into a large beaker filled with 300-400mL of distilled water, and then add 5mL of hydrogen peroxide. The product GNR was obtained by centrifugation.
[0047] (2) Preparation of graphene nanobelt-supported semiconductor titanium dioxide and bismuth tungstate.
[0048] Weigh 0.3g of titanium dioxide precursor, add 24mL of hydrogen peroxide solution and 5mL of ammonia water, and stir unt...
Embodiment 3
[0051] A three-dimensional photocatalytic material GNR-TiO supported by graphene nanoribbons 2 / C 3 N 4 . Contains C, N, Ti, O, H elements.
[0052] (1) Preparation of graphene nanobelts
[0053]Graphene nanoribbons (GNR) were prepared by the following method: weigh 1.5 g of multi-walled carbon nanotubes, add 150 mL of concentrated sulfuric acid, and stir for 1 h. Under ice-bath conditions, slowly add 9 g of potassium permanganate, and stir for 2 h at 25° C. Then in an oil bath at 75 °C for 80 min. Under ice bath conditions, add 25mL of distilled water, then pour the above solution into a large beaker filled with 300-400mL of distilled water, and then add 5mL of hydrogen peroxide. The product GNR was obtained by centrifugation.
[0054] (2) Preparation of graphene nanobelt-supported semiconductor titanium dioxide and carbon nitride
[0055] Weigh 0.3g of titanium dioxide precursor, add 24mL of hydrogen peroxide solution and 5mL of ammonia water, and stir until the solu...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com