Synthesis method of desogestrel drug intermediate
A desogestrel and synthesis method technology, applied in the directions of steroids, organic chemistry, etc., can solve the problems of large environmental pollution, low purity, low yield, etc., and achieves low equipment investment, high purity, and high yield. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
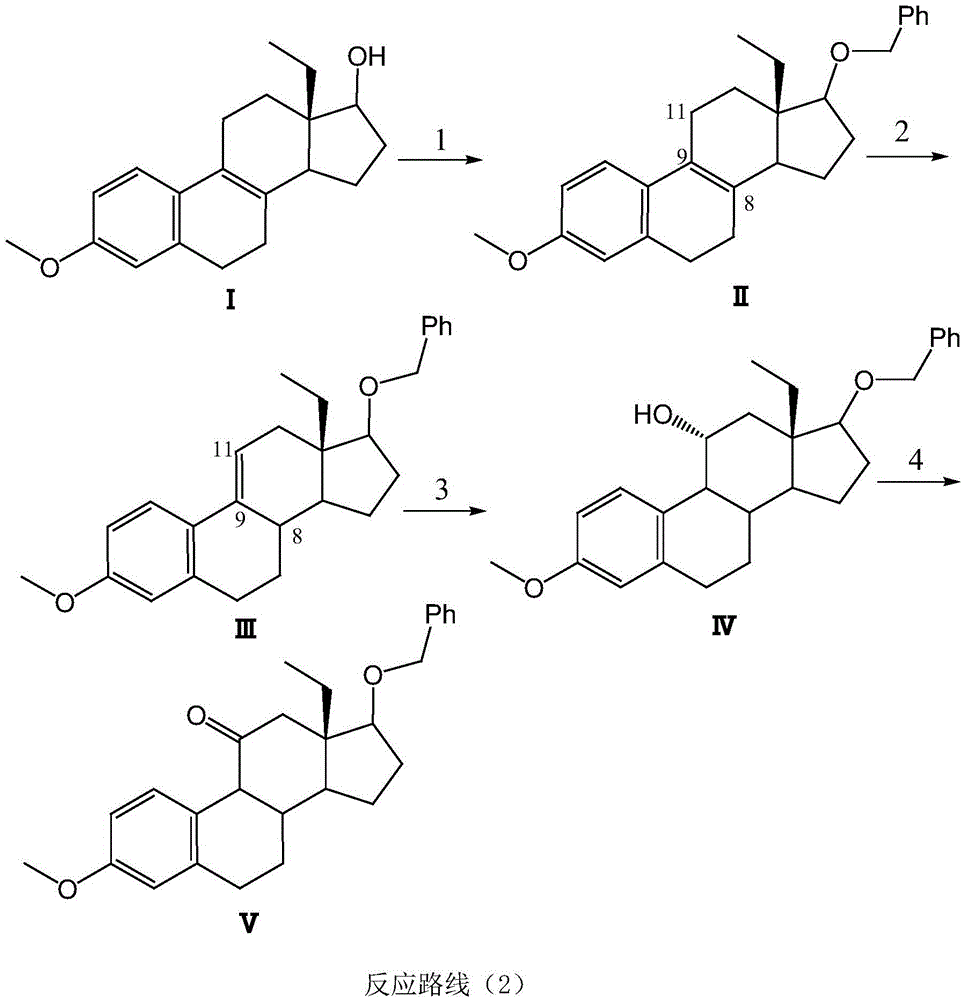
[0019] Example 1: 17β-benzyloxy-D-13β-ethyl-3-methoxyestr-1,3,5(10),8(9)-tetraene (compound II)
[0020]
[0021] Under ice bath, 0.45g of sodium hydride (1.5mmol) was added to 5mL of dimethylformamide solution, and the dried compound I (0.42g, 1.2mmol) was dissolved in 5mL of dry tetrahydrofuran. Add the solution of compound I dropwise to the above solution within 40 minutes, then add benzyl bromide (2.0mmol) dropwise, and the dropwise addition is completed in 30 minutes, and the mixture is stirred at 20-30 degrees Celsius for 20 hours under the protection of nitrogen. , add 30mL of water to dilute, stir to precipitate solid, filter and wash the filter cake with water, grind the solid with cyclohexane, filter and dry to obtain 0.55g of compound II as a white solid with a yield of 95%. Example 2: 17β-benzyloxy-D-13β-ethyl-3-methoxyestr-1,3,5(10),9(11)-tetraene (compound III)
Embodiment 2
[0021] Under ice bath, 0.45g of sodium hydride (1.5mmol) was added to 5mL of dimethylformamide solution, and the dried compound I (0.42g, 1.2mmol) was dissolved in 5mL of dry tetrahydrofuran. Add the solution of compound I dropwise to the above solution within 40 minutes, then add benzyl bromide (2.0mmol) dropwise, and the dropwise addition is completed in 30 minutes, and the mixture is stirred at 20-30 degrees Celsius for 20 hours under the protection of nitrogen. , add 30mL of water to dilute, stir to precipitate solid, filter and wash the filter cake with water, grind the solid with cyclohexane, filter and dry to obtain 0.55g of compound II as a white solid with a yield of 95%. Example 2: 17β-benzyloxy-D-13β-ethyl-3-methoxyestr-1,3,5(10),9(11)-tetraene (compound III)
[0022]
[0023] Compound II (0.51g, 1.3mmol) and p-toluenesulfonic acid (0.09g, 0.307mmol) were dissolved in a mixed solvent of 6mL of glacial acetic acid and 6mL of toluene, and refluxed at 115 degrees Ce...
Embodiment 3
[0024] Example 3: 17β-Benzyloxy-D-13β-Ethyl-11α-Hydroxy-3-methoxyestro-1,3,5(10)-triene (Compound IV)
[0025]
[0026] Compound III (0.42 g, 1.1 mmol) was dissolved in 10 mL of dry THF solvent, followed by the addition of 1M BH 3 .THF solution 5mL, under the protection of nitrogen, react at 25 degrees Celsius for 4 hours, slowly add 0.5mL of water dropwise, then add 30% aqueous sodium hydroxide solution 3mL and 30% hydrogen peroxide 3mL, the reaction solution continues to react at 25 degrees Celsius for 1 hour After the reaction, 25 mL of water was added, the reaction solution was extracted three times with ether (45 mL), the organic phase was washed with saturated sodium bisulfite solution, dried over anhydrous sodium sulfate and distilled under reduced pressure to obtain 0.41 g of compound IV with a yield of 90%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com