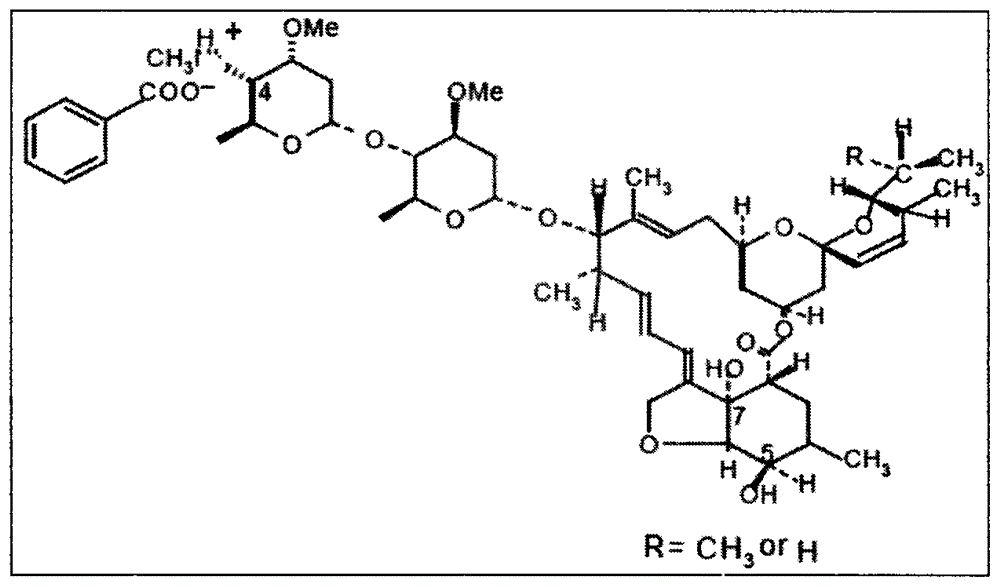
Method for preparing emamectin benzoate
A technology of emamectin salt and methylamino group, applied in the field of optimization research of emamectin salt production method, can solve problems such as unfavorable large-scale production, and achieve the effects of improving yield, easy purification and high selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 15
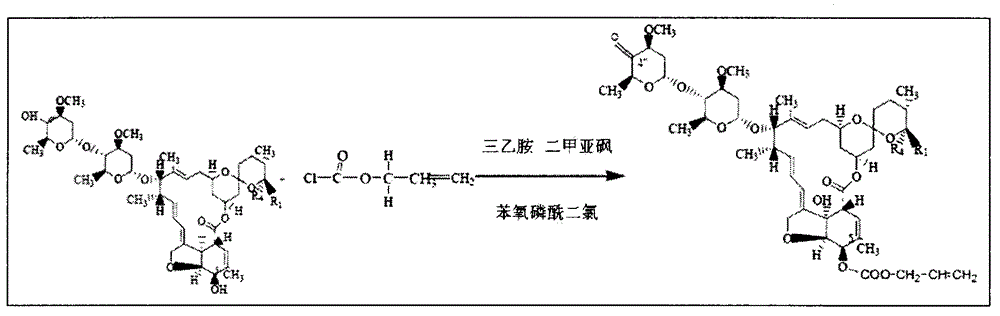
[0026] Embodiment 15-position hydroxyl protection, 4 "-position hydroxyl oxidation
[0027] Add 10g of Abamectin and 50ml of dichloromethane into a three-necked flask with a condenser tube and a thermometer, stir to make it dissolve completely, then add triethylamine and dimethylmethylene containing 50% p-methoxyphenol methyl chloride 30ml of sulfone, heated to 60±2°C, kept warm for 3h, then concentrated, lowered to room temperature, and left for the next reaction.
Embodiment 2
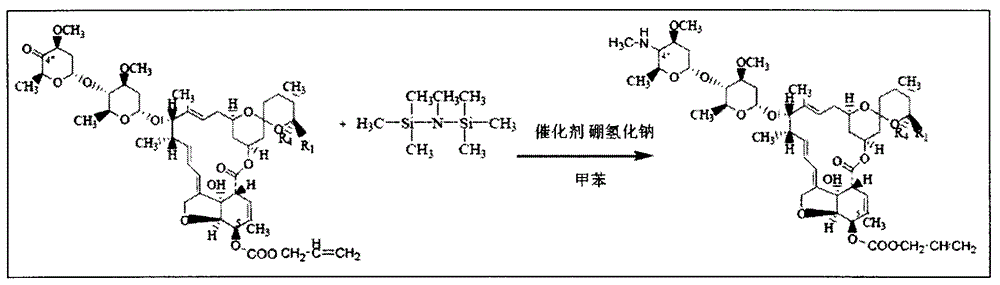
[0028] Embodiment 2 ammoniation reduction reaction
[0029] Add 80ml of methylaminoalcohol solution to the concentrated solution of Example 1, then add 0.05mg of manganese catalyst, heat up to 85±2°C, keep warm for 4h, and obtain 5-O-protecting group-4″-methylamino - The reaction solution of Abamectin was directly used for the next reaction without processing.
Embodiment 3
[0030] Embodiment 3 deprotection reaction
[0031] Add hydrochloric acid to the reaction solution containing 5-O-protecting group-4″-methylamino-abamectin, adjust the pH to 2-3, DDQ0.5g, raise the temperature to 110±2°C, and the reaction time is 4h , Obtain 4 "-methylamino-abamectin solution.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com