A kind of effervescent drug producing polylactic acid glycolic acid and its preparation method and application
A technology of lactic acid glycolic acid and glycolic acid, applied in the directions of drug combination, drug delivery, pharmaceutical formulation, etc., can solve problems such as poor tumor effect, achieve good blood compatibility and biocompatibility, high drug utilization rate, low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Weigh 1 g PLGA (molecular weight 10,000 Daltons, LA:GA=1:1, purchased from Jinan Daigang Biotechnology Co., Ltd.), mix it with 2 mL NMP, and stir at room temperature for 12 hours to obtain a clear and transparent solution. Take 0.1g of citric acid and 0.1g of sodium bicarbonate, mix them evenly with the above PLGA solution, and ultrasonically disperse (output power: 500W) at room temperature for 0.5 hours, so that citric acid and sodium bicarbonate are evenly dispersed in the above PLGA solution. Weigh 5mg of Dox (purchased from Beijing Huafeng Pharmaceutical Co., Ltd.), and ultrasonically disperse in the above PLGA / PT dispersion system (output power: 500W) at room temperature for 0.5 hours to obtain PLGA / PT / Dox. Take 50 μL of PLGA / PT / Dox solution and quickly pour it into water. After the solution completely changes phase, the PLGA / PT / Dox scaffold is obtained. The preparation methods of PLGA, PLGA / PT and PLGA / Dox scaffolds are the same as above.
[0030] From figure 1...
Embodiment 2
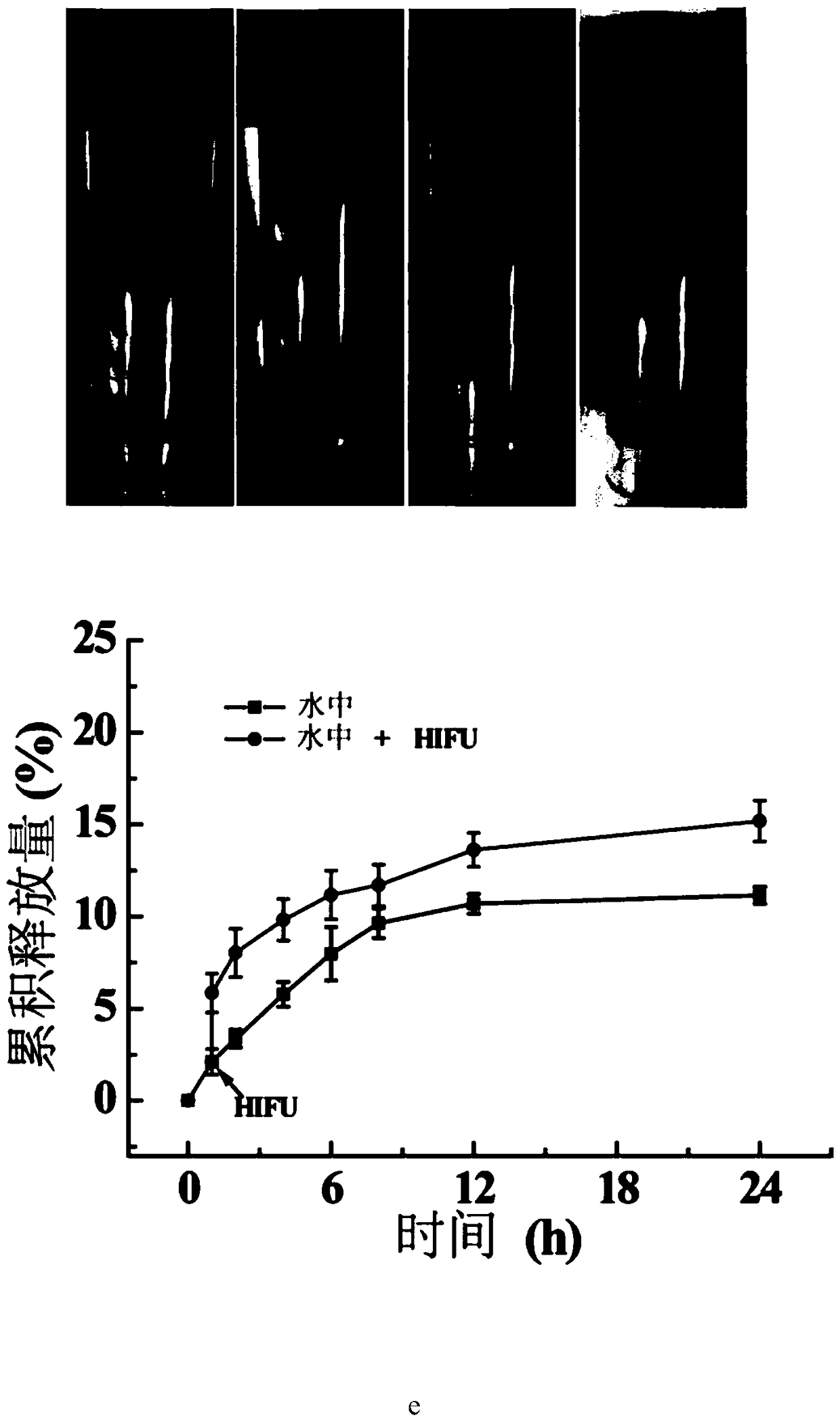
[0032] Take 0.5 mL of PLGA and PLGA / PT / Dox in Example 1, respectively, and inject them into cryovials filled with distilled water. After the phase transition is complete, place the frozen tube in a shaker at 37°C for incubation. At each predetermined time point, pipette 1 mL of DOX-containing distilled water and add 1 mL of the corresponding fresh buffer solution. The concentration of released DOX was determined by the standard curve, the cumulative total release of DOX at different time points was calculated, and the release of DOX by the PLGA / PT / Dox scaffold was analyzed. In order to study the effect of HIFU on the generation speed of air bubbles and drug release speed, PLGA and PLGA / PT / Dox scaffolds were respectively stimulated with HIFU (180W, stimulation time 400ms, interval 200ms, stimulation times 6 times) after incubation for 5min, and the bubbles were observed. and drug release, results such as figure 2 shown. Before and after HIFU stimulation, the PLGA scaffolds ...
Embodiment 3
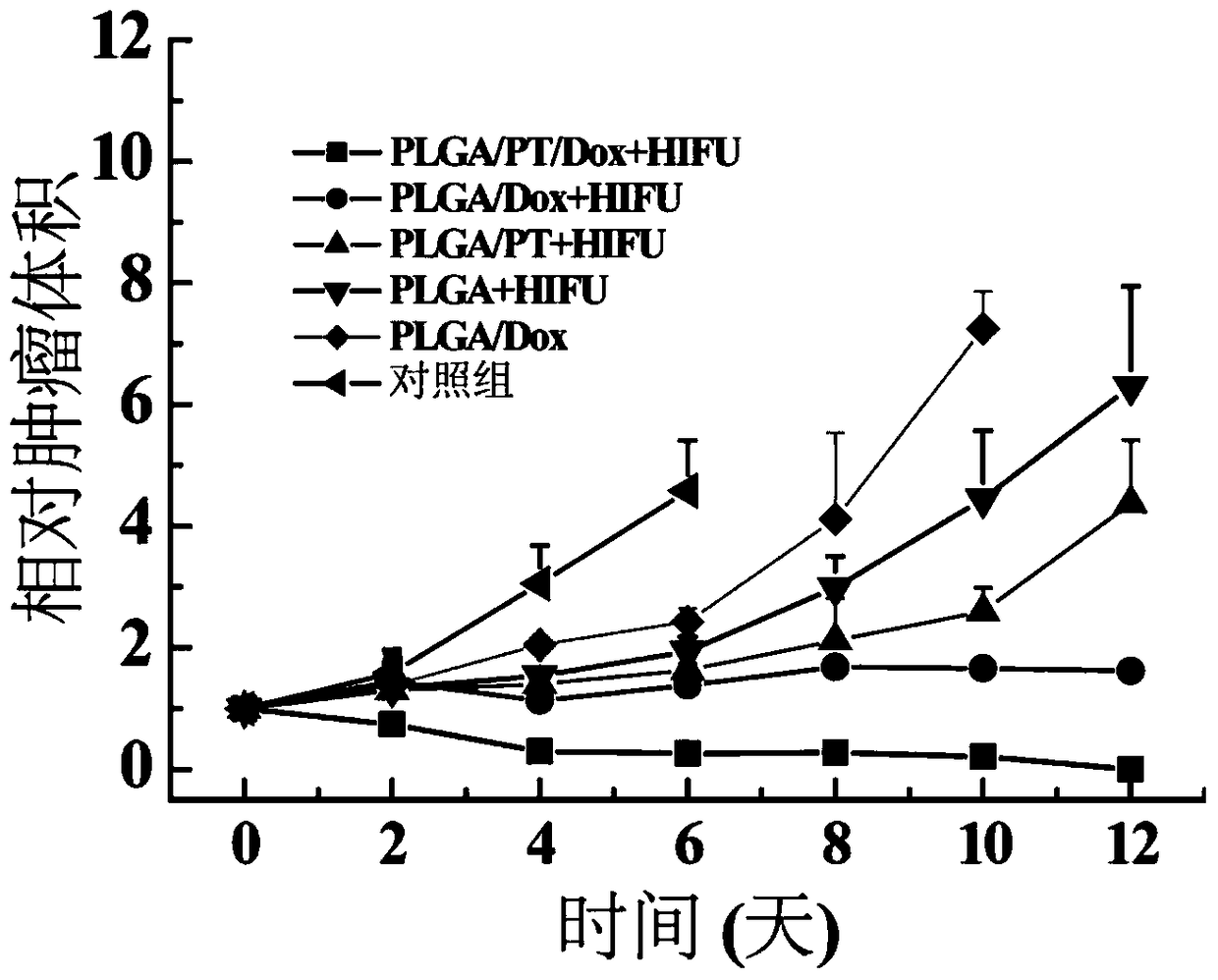
[0034] Eighteen New Zealand white rabbits bearing VX2 tumors (tumor diameter about 1.5-2.0 cm) were randomly divided into 6 groups. Inject ①30 μL normal saline (blank control group); ②30 μL PLGA / Dox; ③30 μL PLGA, supplemented with HIFU stimulation; ④30 μL PLGA / PT, supplemented with HIFU stimulation; ⑤30 μL PLGA / Dox, supplemented with HIFU stimulation; PT / Dox, supplemented by HIFU stimulation (180W, stimulation time 400ms, interval 200ms, stimulation times 6 times). After treatment, the tumor volume of nude mice was recorded every other day. On the 14th day after treatment, blood was taken from the ear veins of each experimental rabbit in group ⑥, and the blood indicators were tested; on the 28th day after treatment, the experimental rabbits in group ⑥ were euthanized, and tissue sections were made for each major organ, and analyzed by H&E staining The pathological changes of each major organ (another consistent healthy rabbit was used as a control).
[0035] The experimental...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com