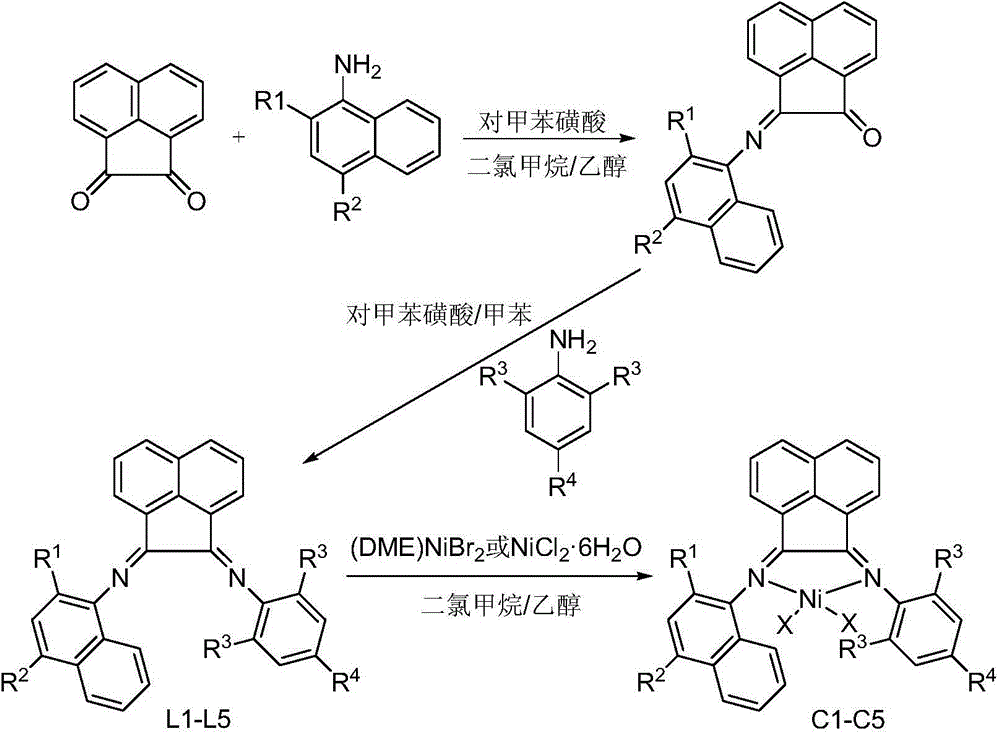
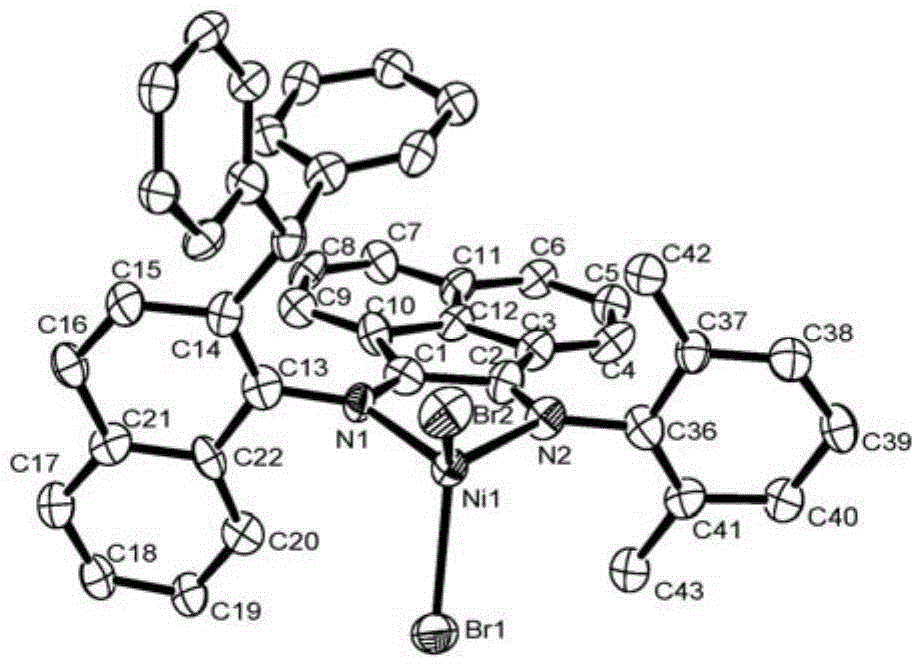
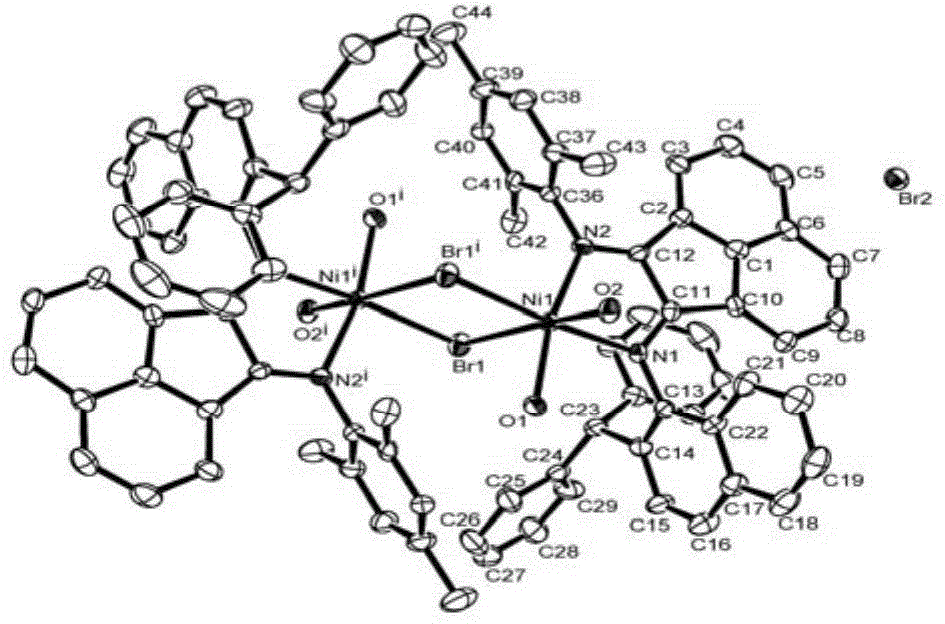
Naphthyl-substituted asymmetric acenaphthenediimine nickel complexes, and preparing method and applications thereof
A technology of metal complexes and ligand compounds, applied in the preparation of imino compounds, nickel organic compounds, organic chemistry, etc., can solve the problems of structural shortage and structural excess.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0091] Embodiment 1, preparation 2-two (phenyl) methylnaphthylamines
[0092] 1-naphthylamine (10g, 63.84mmol), di(phenyl)methanol (8.84g, 34.92mmol), anhydrous ZnCl 2 (5g, 36.68mmol) were added to round bottom flasks respectively, heated in an oil bath, and the temperature was controlled at about 150°C. Reaction 8h. After stopping the heating and cooling, DCM was added to dissolve, filtered through diatomaceous earth, and the solvent was removed, and the residue was subjected to silica gel column chromatography with a mixed solvent of petroleum ether and ethyl acetate at a volume ratio of 100:1. The eluted fraction was detected by a thin-layer silica gel plate, and the solvent was removed to obtain 2.66 g of a purple powder, with a yield of 18.6%.
[0093] The structural confirmation data are as follows: 1 HNMR (400MHz, CDCl 3 ,TMS):δ7.80-7.89(m,2H),7.47-7.49(m,2H),7.29-7.39(m,8H),7.23(d,J=7.2Hz,5H),6.96(d,J =8.4Hz, 1H), 5.79(s, 1H), 3.98(s, 2H).
[0094] 13 CNMR (100M...
Embodiment 2
[0095] Embodiment 2, preparation 1-[2-two (phenyl) methyl naphthylimino] acenaphthylenone
[0096] Acenaphthenequinone (0.73g, 4mmol) and 2-di(phenyl)methylnaphthylamine (1.24g, 4mmol) were dissolved in a mixed solvent of an appropriate amount of ethanol and dichloromethane (the volume ratio of the two was 20:1), and added p-toluenesulfonic acid (0.015g, 0.08mmol), stirred at room temperature for 24h. The solvent was removed, and the residue was subjected to silica gel column chromatography with a mixed solvent of petroleum ether and ethyl acetate at a volume ratio of 80:1. The eluted fraction was detected by a thin-layer silica gel plate, and the solvent was removed to obtain 1.5 g of red powder with a yield of 53%.
[0097] The structural confirmation data are as follows: 1 HNMR (400MHz, CDCl 3 ,TMS): δ7.90(d,J=8.0Hz,1H),7.82–7.76(m,2H),7.71(d,J=8.4Hz,1H),7.63(d,J=8.0Hz,1H) ,7.47(t,J=8.0Hz,2H),7.35–7.04(m,9H),6.99(d,J=8.0Hz,1H),6.85(t,J=8.0Hz,1H),6.47(t, J=7.6Hz, 2H), 5...
Embodiment 3
[0099] Example 3, Preparation of 1-[2-bis(phenyl)methylnaphthalene imino]-2-(2,6-dimethylphenylimino)acenaphthylenene [L1]
[0100] 1-[2-di(phenyl)methylnaphthylimino]acenaphthylenone (3.06g, 6.46mmol) and 2,6-dimethylaniline (0.86g, 7.10mmol) were dissolved in 100mL toluene solution, added P-toluenesulfonic acid (0.03g, 0.13mmol), heated to reflux, reacted for 8h. The solvent toluene was removed, and the residue was subjected to silica gel column chromatography with a mixed solvent of petroleum ether and ethyl acetate at a volume ratio of 200:1. The eluted fraction was detected by a thin-layer silica gel plate, and the solvent was removed to obtain 0.17 g of a red solid, yield: 4.7%. Melting point: 160°C.
[0101] The structure confirmation data are as follows: FT-IR(KBr,cm -1 ):3054(w), 2968(w), 1663(w), 1637(m), 1592(s), 1493(s), 1372(s), 1274(m), 1152(w), 1032(m ), 918(w), 829(m), 778(vs), 745(vs), 696(vs). 1 HNMR (400MHz, CDCl 3 ,TMS): δ7.89(d,J=8.0Hz,1H),7.77–7.74(...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com