Photocatalyst using reducing organic compound
A technology of photocatalysts and organic substances, applied in organic compound/hydride/coordination complex catalysts, chemicals for biological control, physical/chemical process catalysts, etc., can solve limited applicable occasions, difficult processing technology, oxidation Titanium safety doubts and other issues, to achieve the effect of high safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0146] [Example 1: "Raw materials containing reducing organic matter"]
[0147] By verifying whether there is activity of reducing ferric iron to ferrous iron, it can be judged whether various raw materials are reducing organic matter.
[0148] 【(1) "Verification of Iron Reducing Ability"】
[0149] For each 100 parts by weight (dry weight conversion) of each raw material shown in Table 1, prepare each aqueous solution (0.1wt% each raw material, 0.1wt% ferric chloride solution) and allowed to stand at room temperature for a few minutes. Thereafter, 2 g / L of bipyridyl groups and 100 g / L of acetic acid were added and mixed to each aqueous solution, and the presence or absence of a color reaction was examined. Among them, the bipyridyl group is a substance that remains colorless without reacting with trivalent iron, but turns red when it reacts with divalent iron. Used in the detection of ferrous iron. In addition, as a control, a 0.1 wt% iron (III) chloride aqueous solution w...
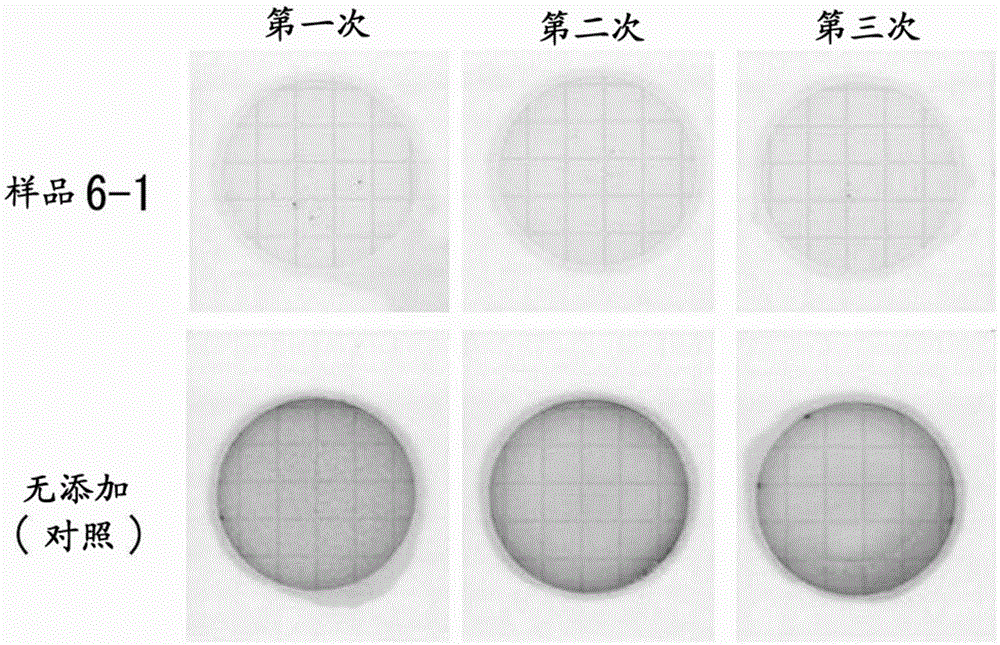
Embodiment 2
[0157] [Example 2: "Wavelength for Exciting Photocatalyst Activity"]
[0158] The 'reaction product of reducing organic matter and iron' was prepared using tea or coffee grounds as a raw material, and the photocatalytic activity of the material was investigated.
[0159] 【(1) "Measurement of photocatalyst activity"】
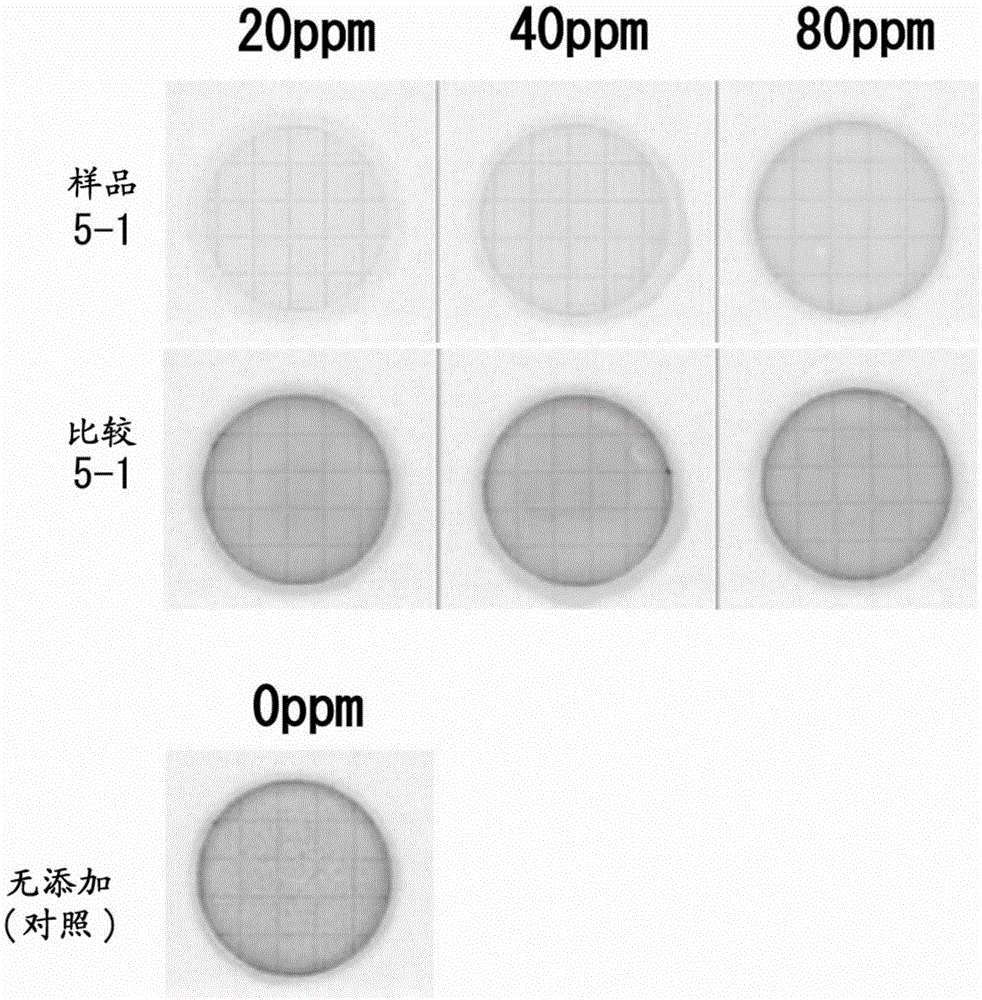
[0160] Mix 4 parts by weight of ferric chloride in terms of iron element with respect to 100 parts by weight (dry weight conversion) of tea grounds (residue of hot water extraction of tea leaves) or coffee grounds (residue of hot water extraction of ground roasted coffee beans) (III)(FeCl 3 ), add water twice the weight of the total amount of the two, and heat-treat at 98° C. for 1 hour to obtain a reaction product. The filtrates obtained by filtration were respectively referred to as "tea leaf component-iron" (sample 2-1) or "roasted coffee bean component-iron" (sample 2-2). In addition, as a comparative sample, titanium oxide (TiO 2 : titanium (IV) oxide an...
Embodiment 3
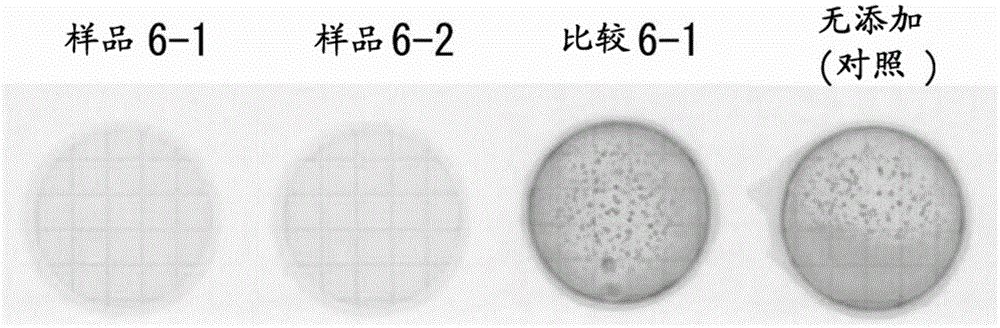
[0178] [Example 3: "Discussion with various reducing organic substances"]
[0179] When preparing reaction products of various reducing organic substances and iron, whether or not photocatalytic activity can be confirmed is also examined.
[0180] 【(1) "Measurement of photocatalyst activity"】
[0181] 4 parts by weight of iron (III) chloride in terms of iron element were mixed with 100 parts by weight (dry weight conversion) of each of ascorbic acid, grape polyphenols, catechin, chlorogenic acid, caffeic acid, tannin, or furfur vinegar solution (FeCl 3 ), add water twice the weight of the total amount of the two, and heat-treat at 98° C. for 1 hour to obtain the reaction products shown in Table 3 (samples 3-1 to 3-7). In addition, as a comparative sample, titanium oxide (TiO 2 : titanium (IV) oxide anatase type, particle size 100 to 300 nm, manufactured by WAKO) (comparison 3-1).
[0182] Next, a plurality of 3.5 ppm basic fuchsin aqueous solutions were prepared to add eac...
PUM
| Property | Measurement | Unit |
|---|---|---|
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com