Preparation method of tenofovir disoproxil fumarate oral tablets
A technology of tenofovir fumarate and disoproxil, which is applied in the directions of non-active ingredient medical preparations, medical preparations containing active ingredients, and pill delivery, etc. It can reduce the difficulty of swallowing, increase the drug dissolution rate, and reduce the particle size.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
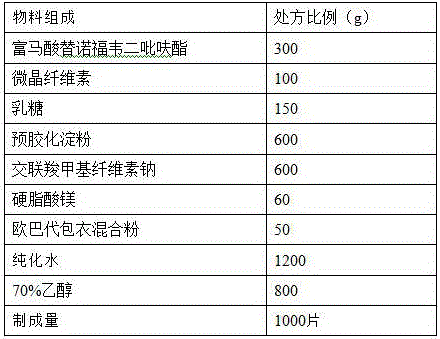
[0015] prescription:
[0016]
[0017] Preparation method: (1) Weigh each material separately, micronize, pass tenofovir disoproxil fumarate raw material through a 100-mesh sieve, microcrystalline cellulose, lactose, pregelatinized starch, cross-linked carboxymethyl Sodium cellulose is passed through a 80-mesh sieve, magnesium stearate and Opadry coating mixed powder are passed through a 40-mesh sieve for subsequent use;
[0018] (2) Take the prescription amount of 60% ethanol (v / v) solution, add the above prescription amount of micronized pregelatinized starch, and prepare a pregelatinized starch suspension with a concentration of 10% (w / v), and set aside ;
[0019] (3) Mix tenofovir disoproxil fumarate, lactose, microcrystalline cellulose, and croscarmellose sodium (75% prescription amount) evenly with the suspension treated in step (2), Soft materials are prepared in a fluidized bed, granulated through 18 mesh, dried at an air inlet temperature of 45°C, and the moistur...
Embodiment 2
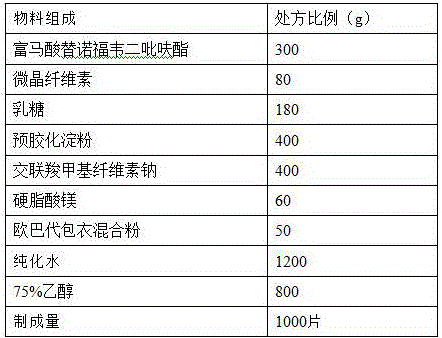
[0024] prescription:
[0025]
[0026] Preparation method: (1) Weigh each material separately, micronize, pass tenofovir disoproxil fumarate raw material through a 100-mesh sieve, microcrystalline cellulose, lactose, pregelatinized starch, cross-linked carboxymethyl Sodium cellulose is passed through a 80-mesh sieve, magnesium stearate and Opadry coating mixed powder are passed through a 40-mesh sieve for subsequent use;
[0027] (2) Take the prescription amount of 75% ethanol (v / v) solution, add the above prescription amount of micronized pregelatinized starch, and prepare a pregelatinized starch suspension with a concentration of 15% (w / v), and set aside ;
[0028] (3) Mix tenofovir disoproxil fumarate, lactose, microcrystalline cellulose, and croscarmellose sodium (75% prescription amount) evenly with the suspension treated in step (2), Soft materials are prepared by fluidized bed, granulated through 30 mesh, dried at air inlet temperature of 50°C, and moisture content...
Embodiment 3
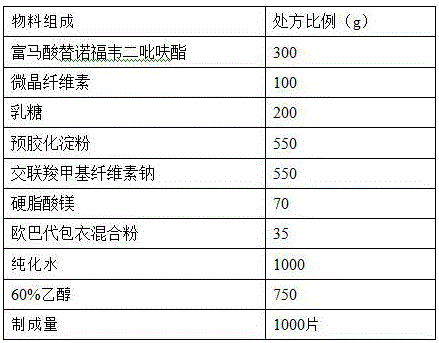
[0033] prescription:
[0034]
[0035] Preparation method: (1) Weigh each material separately, micronize, pass tenofovir disoproxil fumarate raw material through a 100-mesh sieve, microcrystalline cellulose, lactose, pregelatinized starch, cross-linked carboxymethyl Sodium cellulose is passed through a 80-mesh sieve, magnesium stearate and Opadry coating mixed powder are passed through a 40-mesh sieve for subsequent use;
[0036] (2) Take the 70% ethanol (v / v) solution of the prescription amount, add the micronized pregelatinized starch of the above prescription amount, and prepare a pregelatinized starch suspension with a concentration of 20% (w / v), and set aside ;
[0037] (3) Mix tenofovir disoproxil fumarate, lactose, microcrystalline cellulose, and croscarmellose sodium (75% prescription amount) evenly with the suspension treated in step (2), Fluidized bed preparation of soft materials, granulation through 20 mesh, air inlet temperature 55 ℃ drying, control moisture ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com