Endotoxin adsorption material for blood purification and preparation method and application of endotoxin adsorption material for blood purification
A technology for blood purification and adsorption materials, applied in chemical instruments and methods, blood circulation treatment, suction devices, etc., can solve the problems of large batch differences, many reaction steps, and unstable performance of adsorption material products, and improve the binding efficiency. , The effect of simplifying the preparation process and reducing the difference between batches
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] 1. Reaction of agarose gel with ethylene glycol bisglycidyl ether
[0042] (1) Carrier activation: Add 0.5 L of agarose gel (Cl-6B, particle size 300-600 μm) and 1.0 mol / L NaOH aqueous solution (containing 5 mg / mL of NaBH) into a 2.0 L reactor 4 solution) 20mL, mixed evenly, added 500mL of ethylene glycol bisglycidyl ether, placed in a constant temperature shaker, reacted at 40°C for 5h, and ended the reaction to obtain an activated carrier;
[0043] (2) Filter the activated carrier obtained in step (1), and use 20 times the volume of distilled water, ethanol / water (1:1 by volume ratio), absolute ethanol, ethanol / water (1:1 by volume ratio) and Rinse with distilled water;
[0044] Store the activated carrier at 4°C for later use; use the sodium thiosulfate method to detect the number of epoxy groups in the gel activated by this method, and measure at least 50 μmol of epoxy active groups per milliliter, which is recorded as E1-1.
[0045] 2. Reaction of agarose gel wi...
Embodiment 2
[0062] Synthesis of endotoxin-absorbing materials
[0063] 1. Reaction of active agarose gel (Cl-6B) with polylysine and glycine to synthesize endotoxin adsorption material for blood purification
[0064] Take 10 g of active agarose gel (E1-1) and add 20 mL of an aqueous solution containing 1.0 g of polylysine and 0.2 g of glycine, adjust the pH to 12, and react at 60°C for 24 hours; wash with distilled water after the reaction; then add 20mL of 1.0mol / L ethanolamine solution, adjust the pH to 8.0, capping reaction, shaking reaction at 37°C for 4 hours; after the reaction is completed, wash with distilled water to obtain endotoxin adsorption material E1-1-1 for blood purification.
[0065] 2. Reaction of active agarose gel (Cl-6B) and arginine to synthesize endotoxin adsorption material for blood purification
[0066] Take 10g of active agarose gel (E1-1) and add 60mL of aqueous solution containing 0.8g of arginine, adjust the pH value to 5.0, and react at 37°C for 24 hours; ...
Embodiment 3
[0098] Adsorption endotoxin test
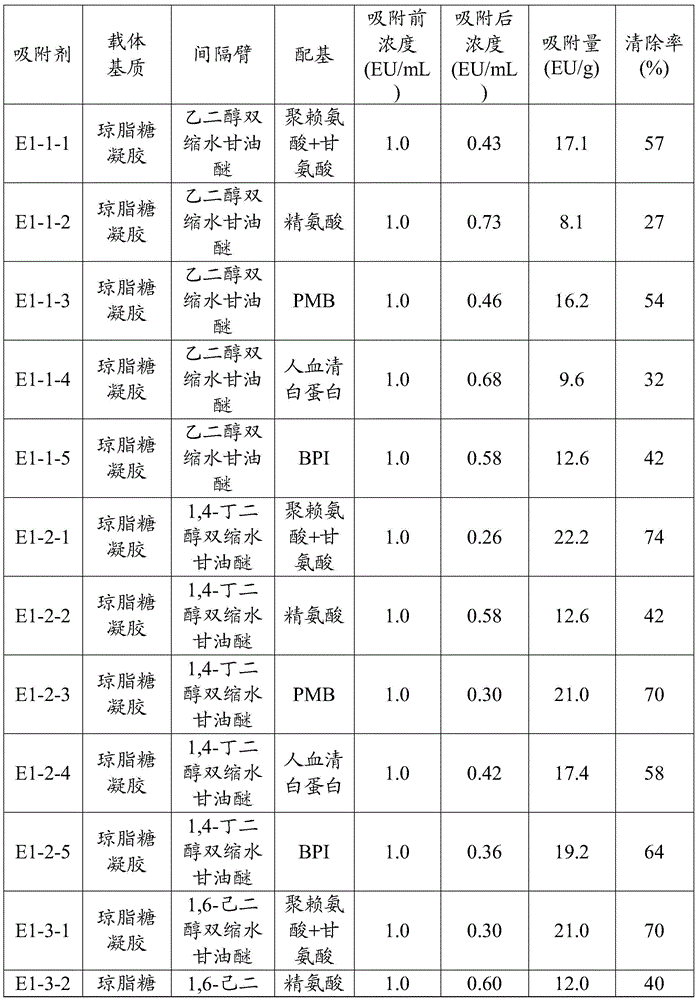
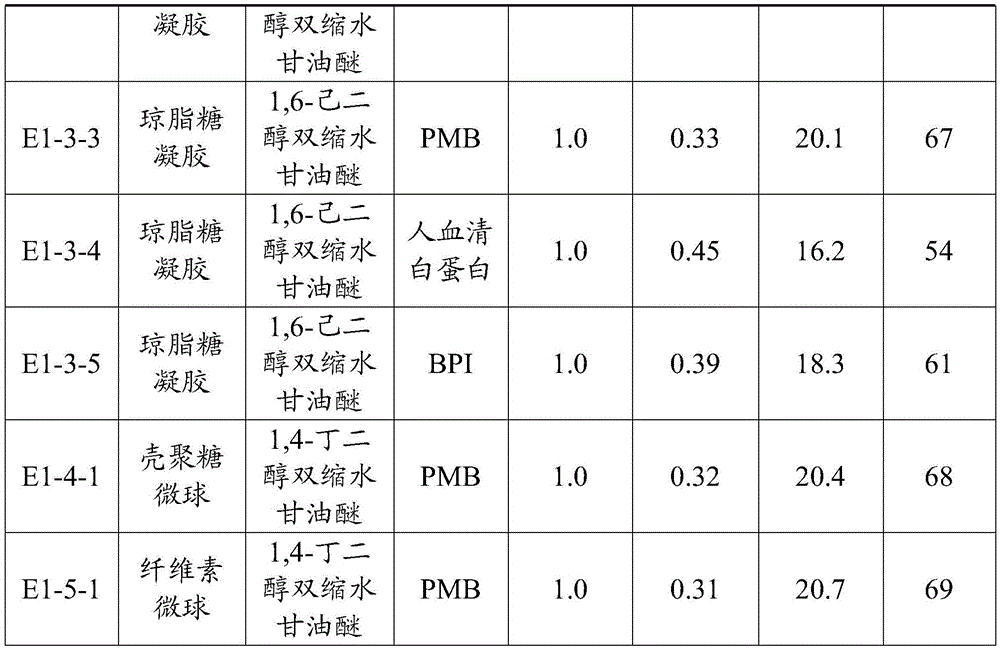
[0099] The endotoxin concentration of patients with endotoxemia is generally less than 1EU / mL, and the initial concentration of adsorption is set to be 1EU / mL in the experiment. Take a 10 mL pyrogen-free Erlenmeyer flask, add 0.2 g of the endotoxin adsorption material for blood purification prepared in Example 2, and then add 6 mL of plasma containing endotoxin 1 EU / mL, and oscillate for 2 hours (temperature 37 ° C, oscillating Speed 180rpm), then use the dynamic turbidity method to measure the endotoxin concentration after adsorption, and calculate its adsorption capacity and clearance rate by the adsorbent, the results are shown in Table 1.
[0100] Adsorption capacity and clearance rate of endotoxin by each endotoxin adsorption material for blood purification in Example 2 in Table 1
[0101]
[0102]
[0103]It can be seen from the test results that the endotoxin adsorption materials for blood purification in the examples of the p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com