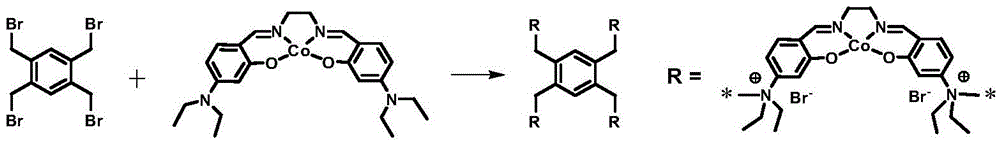
Dual-function catalyst for preparing cyclic carbonates through cycloaddition of CO2 and epoxy compound and preparation method thereof
A bifunctional catalyst and epoxy compound technology, which is applied in the field of environmental science and fine organic chemical industry, can solve problems such as the greenhouse effect, and achieve the effects of simple preparation, high catalytic activity, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
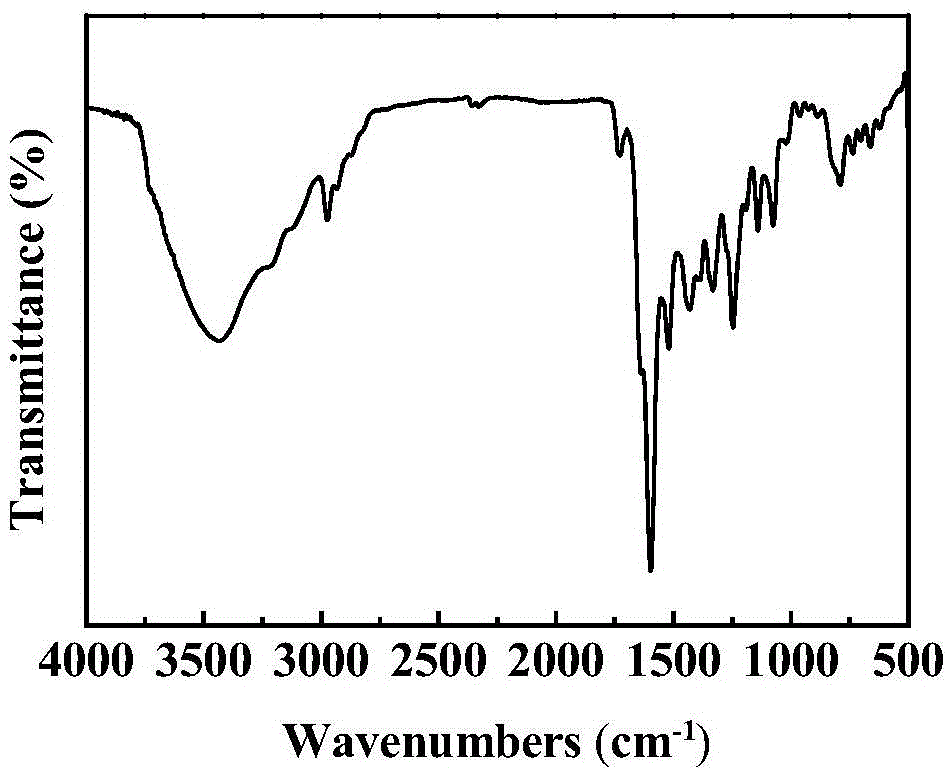
[0015] Diethylamine and 4-diethylamino salicylaldehyde were added to 30mL ethanol solvent according to the molar ratio of 1:2, and after reflux and stirring for 24h, the mixture was lowered to room temperature and allowed to stand for 48h, the yellow crystals were filtered out, and washed with ethanol Washing three times, drying in vacuo at 50°C for 24 hours to obtain the Salen ligand, then adding the Salen ligand and cobalt acetate tetrahydrate to 30 mL of a mixed solvent of toluene and methanol in a molar ratio of 1:1 (volume ratio of 1:1), After the above mixed solution was stirred at 60°C for 24h, tetrabromotoluene was added thereto (1 / 2 of the molar weight of the Salen ligand), and the stirring was continued for 24h, the solid product was filtered out, washed twice with ethyl acetate, and then heated at 60°C Dry under vacuum for 24 hours to obtain the bifunctional catalyst TBB-Salen-Co. For its synthetic route, see figure 1 , Infrared characterization spectrum see figu...
Embodiment 2
[0017] Propylene oxide and catalyst TBB-Salen-Co were added into the miniature autoclave according to the mass ratio of 20:1, CO 2 The pressure was 1 MPa, and the reaction was stirred at 70° C. for 6 h to obtain a mixture containing cyclic carbonate products. According to gas chromatographic analysis, the conversion rate of propylene oxide is 95%, and the selectivity of cyclocarbonate product is 100%.
Embodiment 3
[0019] Add epichlorohydrin and catalyst TBB-Salen-Co into the miniature autoclave according to the mass ratio of 20:1, CO 2 The pressure was 1 MPa, and the reaction was stirred at 80° C. for 8 h to obtain a mixture containing cyclic carbonate products. According to gas chromatographic analysis, the conversion rate of epichlorohydrin is 90%, and the selectivity of cyclocarbonate product is 95%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com