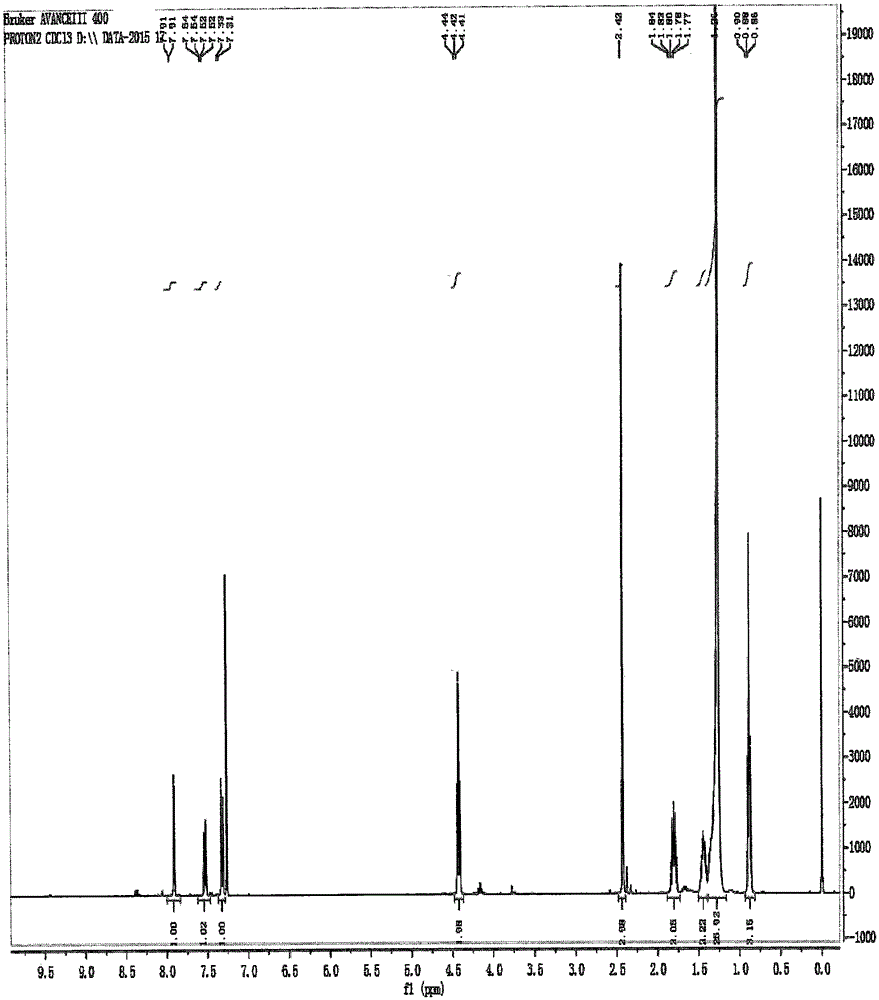
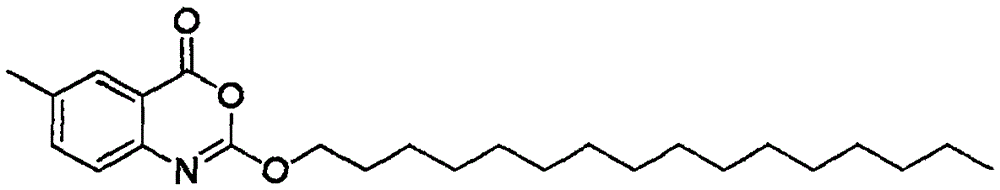
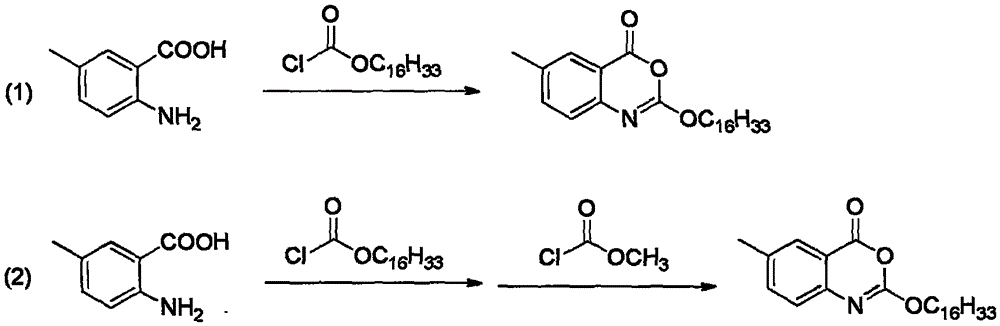
Preparation method of cetilistat
A technology of new listat and compounds, applied in the field of drug preparation, can solve the problems of inconvenient industrial production and achieve the effect of cheap and easy-to-obtain reagents
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] The preparation of embodiment 1 compound C
[0052]Disperse compound A (15.1g) with 250mL dichloromethane in a 500mL three-necked flask, add compound B (30.5g) under stirring, add pyridine (16.1mL) to the system under ice-water bath, drop it, and naturally rise to room temperature for reaction .
[0053] TLC (n-hexane: ethyl acetate: glacial acetic acid = 5:1:0.1) monitors the progress of the reaction. After the reaction is complete, add 80 mL of water to wash, extract the aqueous layer with an appropriate amount of dichloromethane, combine the organic layers, and add dilute hydrochloric acid to the system. Adjust the pH to 1-2, stir in an ice-water bath (0-10°C) to precipitate a solid, filter with suction, wash the filter cake with water, rinse the filter cake with dichloromethane, and dry it with air at 40°C to obtain 38.7 g of off-white solid, yield 92.3%.
Embodiment 2
[0054] The preparation of embodiment 2 compound C
[0055] Disperse compound A (15.1g) with 250mL dichloromethane in a 500mL three-necked flask, add compound B (30.5g) under stirring, add triethylamine (27.7mL) to the system under an ice-water bath, drop it, and naturally rise to React at room temperature.
[0056] TLC (n-hexane: ethyl acetate: glacial acetic acid = 5:1:0.1) monitored the progress of the reaction. After the degree of reaction no longer increased, 80 mL of water was added for washing, and the aqueous layer was extracted with an appropriate amount of dichloromethane. The organic layers were combined and poured into the system. Add dilute hydrochloric acid to adjust the pH to 1-2, stir and precipitate solids in an ice-water bath (0-10°C), filter with suction, wash the filter cake with water, rinse the filter cake with dichloromethane, and blow dry at 40°C to obtain 28.7g off-white Solid, yield 68.4%.
Embodiment 3
[0057] The preparation of embodiment 3 compound C
[0058] Disperse Compound A (15.1g) with 250mL ethyl acetate in a 500mL three-neck flask, add Compound B (30.5g) under stirring, add pyridine (16.1mL) to the system under ice-water bath, drop it, and naturally rise to room temperature for reaction .
[0059] TLC (n-hexane: ethyl acetate: glacial acetic acid = 5:1:0.1) monitors the progress of the reaction. After the reaction is complete, add 80 mL of water to wash, extract the aqueous layer with an appropriate amount of ethyl acetate, combine the organic layers, and add dilute hydrochloric acid to the system. Adjust the pH to 1-2, stir in an ice-water bath (0-10°C) to precipitate a solid, filter with suction, wash the filter cake with water, rinse the filter cake with ethyl acetate, and dry it with air at 40°C to obtain 24.6g of off-white solid, yield 58.7%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com