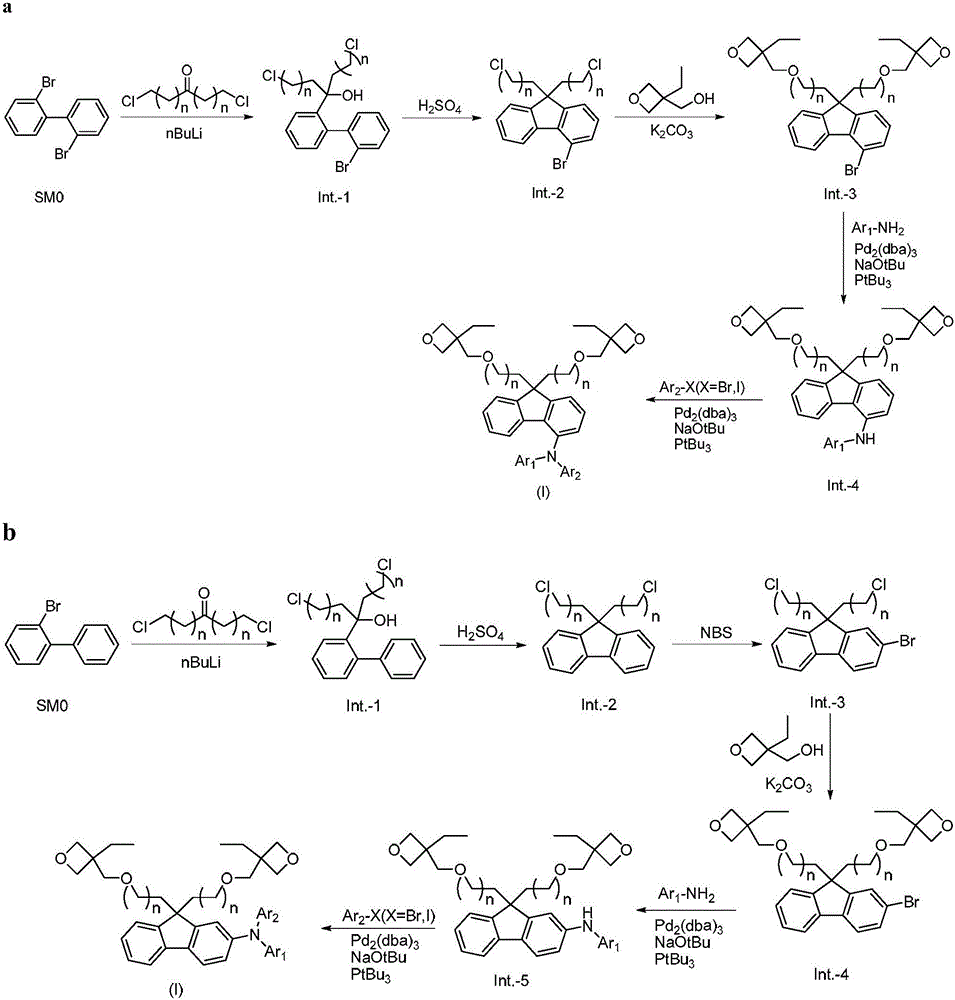
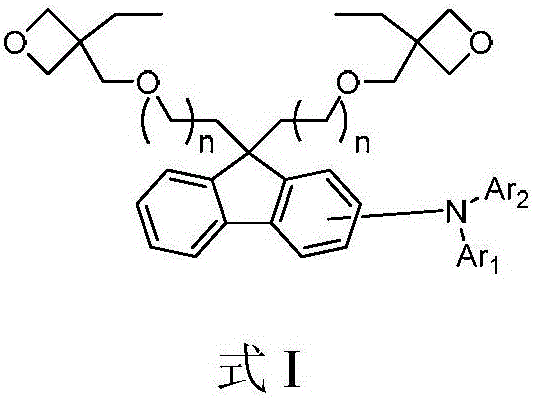
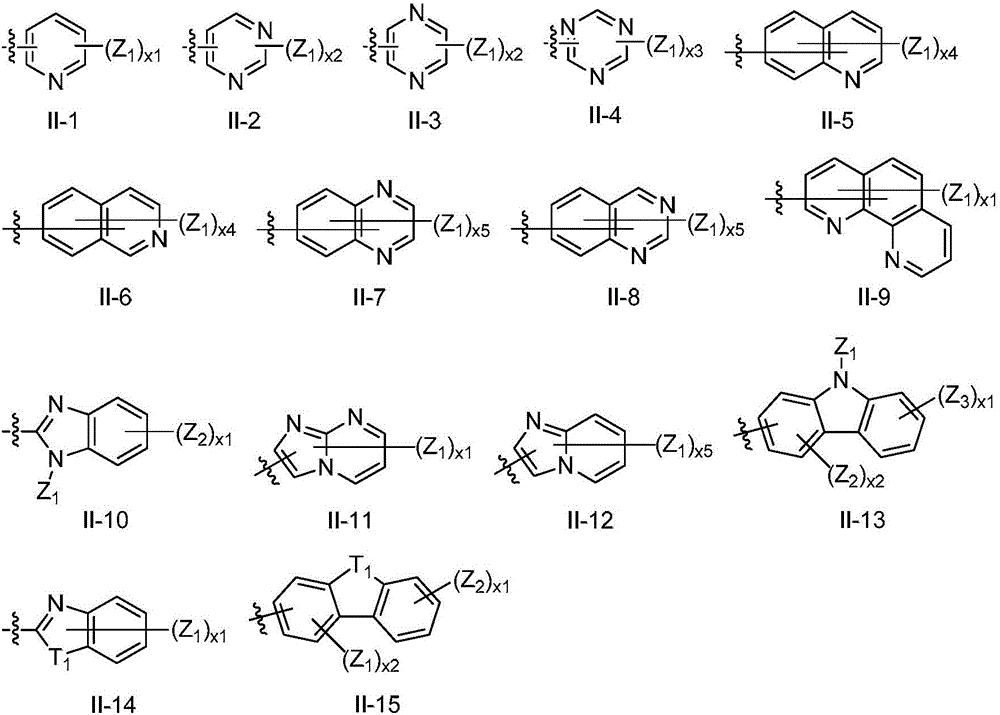
Luminescent material of a series of fluorene derivatives
A technology of light-emitting materials and light-emitting devices, which is applied in the field of materials and can solve problems such as reduced luminous efficiency, unbalanced distribution of electrons and holes, and uneven molecular weight distribution.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0098] Embodiment 1, the preparation of compound 14 (n=2)
[0099]
[0100] The first step: the preparation of intermediate-1
[0101]
[0102] 20g of 2,2'-dibromobiphenyl was dissolved in 400ml of dry tetrahydrofuran, placed in a low-temperature tank under nitrogen protection, cooled to -80°C with liquid nitrogen, and slowly added dropwise to 25ml of 2.5M n-butyllithium- Hexane solution, temperature controlled and stirred at -70°C for 30 minutes, set aside.
[0103] 14g of 1,7-dichloroheptanone was dissolved in 150ml of dry tetrahydrofuran, placed in a low-temperature tank under the protection of nitrogen, cooled to -80°C with liquid nitrogen, and the reaction solution prepared above was slowly added dropwise, and the reaction was stirred for 1 hour, rose to room temperature and stirred for 30 minutes, added 100ml of saturated ammonium chloride aqueous solution to quench the reaction, extracted three times with ethyl acetate, and the organic phase was washed with anhyd...
Embodiment 2
[0123] Embodiment 2, the preparation of compound 93 (n=2)
[0124]
[0125] The first step: the preparation of intermediate-1
[0126]
[0127] 20g of 2-bromobiphenyl was dissolved in 400ml of dry tetrahydrofuran, placed in a low-temperature tank under nitrogen protection, cooled to -80°C with liquid nitrogen, and slowly added dropwise to 34.5ml of 2.5M n-butyllithium-hexane solution , temperature controlled and stirred at -70°C for 30 minutes, set aside.
[0128] Dissolve 19g of 1,7-dichloroheptanone in 150ml of dry tetrahydrofuran, place it in a low-temperature tank under the protection of nitrogen, cool down to -80°C with liquid nitrogen, slowly drop the reaction solution prepared above, and stir for 1 hour, rose to room temperature and stirred for 30 minutes, added 100ml of saturated ammonium chloride aqueous solution to quench the reaction, extracted three times with ethyl acetate, and the organic phase was washed with anhydrous Na 2 SO 4 After drying and filteri...
Embodiment 3
[0151] Embodiment 3, the preparation of compound formula 1~13, 15~87
[0152] Compounds 1-13, 15-87 can be prepared by referring to the preparation method of Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com