PET tracer agent precursor-2-nitroimidazole compound and preparation method thereof
A nitroimidazole and tracer technology, applied in the field of radiopharmaceutical synthesis, can solve the problems of inability to guarantee specific imaging activity, poor image clarity, low target/background value, etc., and achieves rapid tissue penetration, Short imaging time interval, evenly distributed effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: the preparation of NOTA-Nitro1:
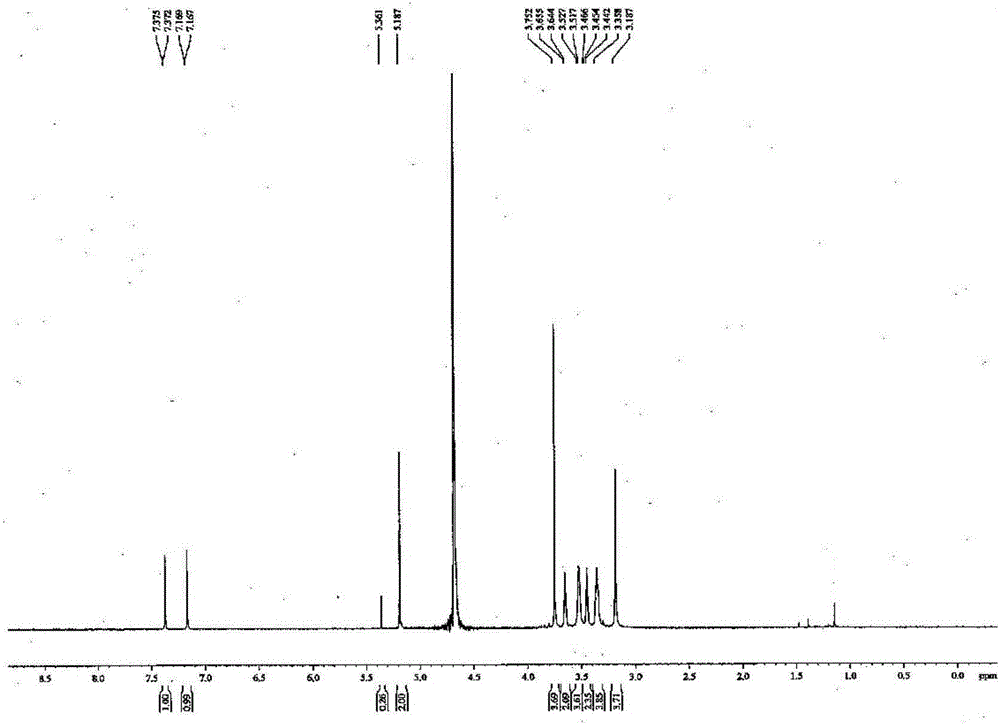
[0030] (1) Preparation of 1,4-bis(tert-butoxycarbonylmethyl)1,4,7-triazacyclononane: tert-butyl bromoacetate (21.4g, 55mmol) was dissolved in CHCl 3 (100mL), at room temperature, add 1,4,7-triazacyclononane (6.5g, 50mmol) in CHCl using a syringe pump for 1h 3 (50mL) solution for 24h. After filtration, the filtrate was evaporated to dryness, 50 mL of distilled water was added, and the pH was adjusted to 3 with 1M HCl. Extracted 3 times with 50 mL ether. The organic layer was evaporated to dryness to obtain the tri-tert-butoxycarbonylmethyl substitution of 1,4,7-triazacyclononane. The aqueous layer was adjusted to pH 11 with 1M NaOH. With 50mLCH 2 Cl 2 Extracted three times, combined the organic phases, added n-hexane for recrystallization, and obtained the disubstituted product 1,4-bis(tert-butoxycarbonylmethyl)1,4,7-triazacyclononane. The yield was 61%.
[0031] (2) Preparation of tert-butyl 2-(2-nitro-1H-imidazol-...
Embodiment 2
[0037] Embodiment 2: the preparation of PET tracer 18F-FAl-NOTA-Nitro1
[0038] (1) Preparation of 18F solution: Proton velocity bombardment of H in a medical accelerator 2 18 get 18 F solution. Using CRC-15R activity meter (CAPINTEC) to measure the radiation dose was 20mCi.
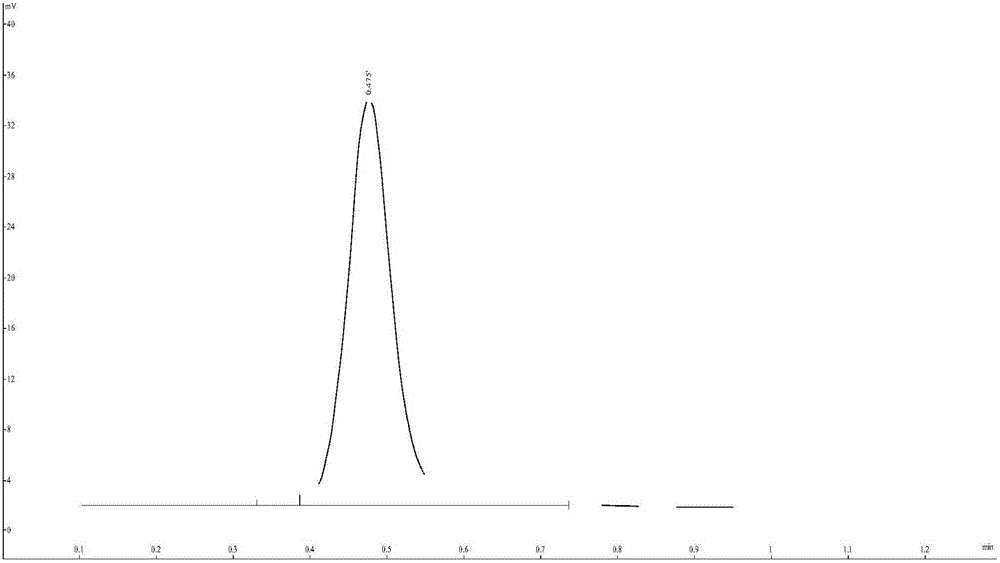
[0039] (2) Preparation of PET tracer 18F-FAl-NOTA-Nitro1: Take the precursor NOTA-Nitro10.1 mg prepared in Example 1 in a vial, add 10 μL of glacial acetic acid, 0.005 mg of aluminum trichloride (acetic acid / acetic acid Dissolve in sodium buffer solution), 200 μL acetonitrile, continue to add 50 μL 18F ionic solution, and react in a closed manner at 100°C for 20 minutes. After the reaction is completed, cool to room temperature and then add ultrapure water, let the reaction solution pressurize through the pre-activated aluminum chloride column, The unreacted 18F ions are adsorbed, the eluate is collected, and the used solution is removed by heating, and the target product is obtained by dissolving wi...
Embodiment 3
[0042] Embodiment 3: MicroPET imaging test
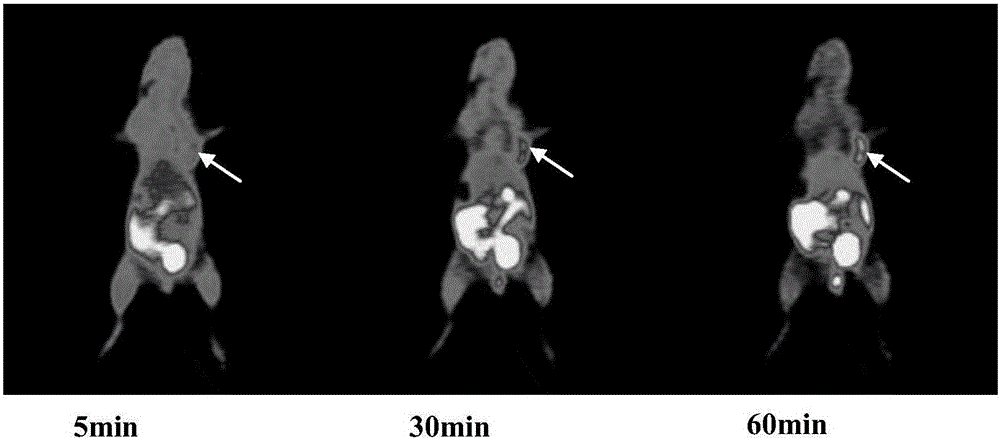
[0043] Model mice bearing human transplanted tumors (ECa109 esophageal carcinoma) were placed on small animal PET beds, anesthetized with isoflurane, and fixed with adhesive tape. The model mice were injected with the tracer physiological saline solution (1.85 MBq, 0.2 ml) prepared in Example 2 above through the tail vein. Dynamic scanning was performed 2 hours after injection, and MicroPET imaging was performed respectively. The results were as follows: image 3 shown.
[0044] The image reconstruction is carried out by two-dimensional ordered subset expectation maximization method. Calculate the radioactivity (MBq / ml) in the tumor, muscle, liver and other organs with the region of interest (ROI) method, and divide the obtained value by the injected dose to obtain the PET tracer uptake value (%ID / g) of each tissue (assumed The tissue density is 1 g / ml). The calculation results are as Figure 4 shown. The target / non-target rat...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com