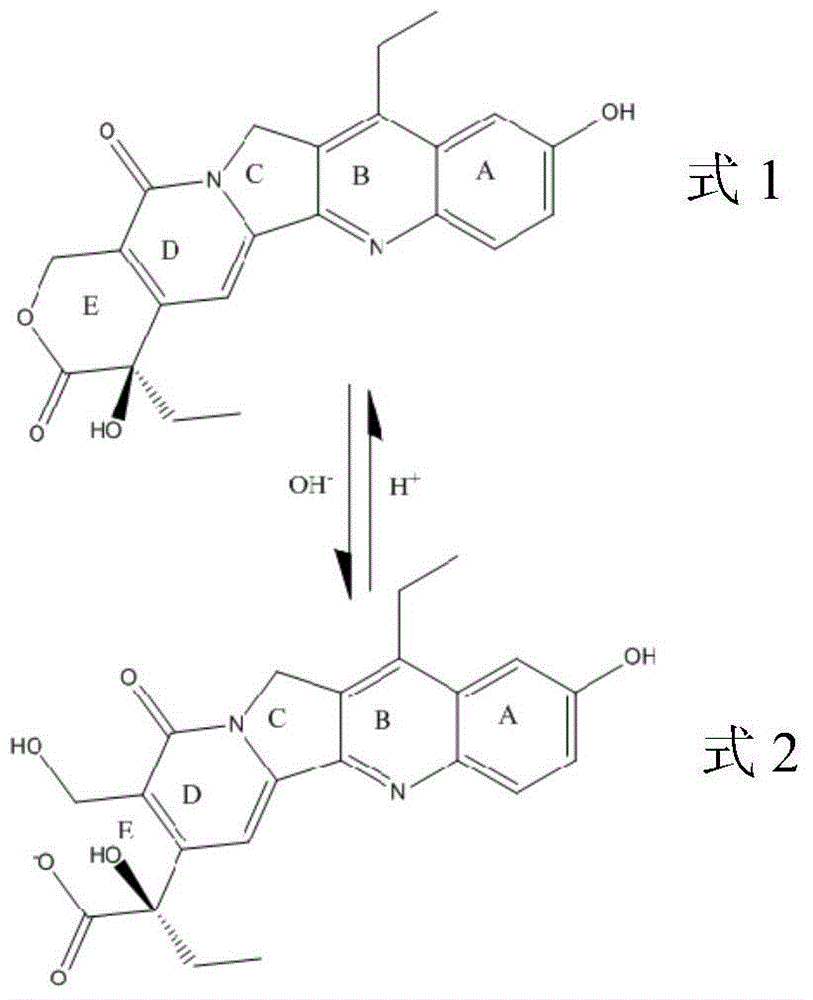
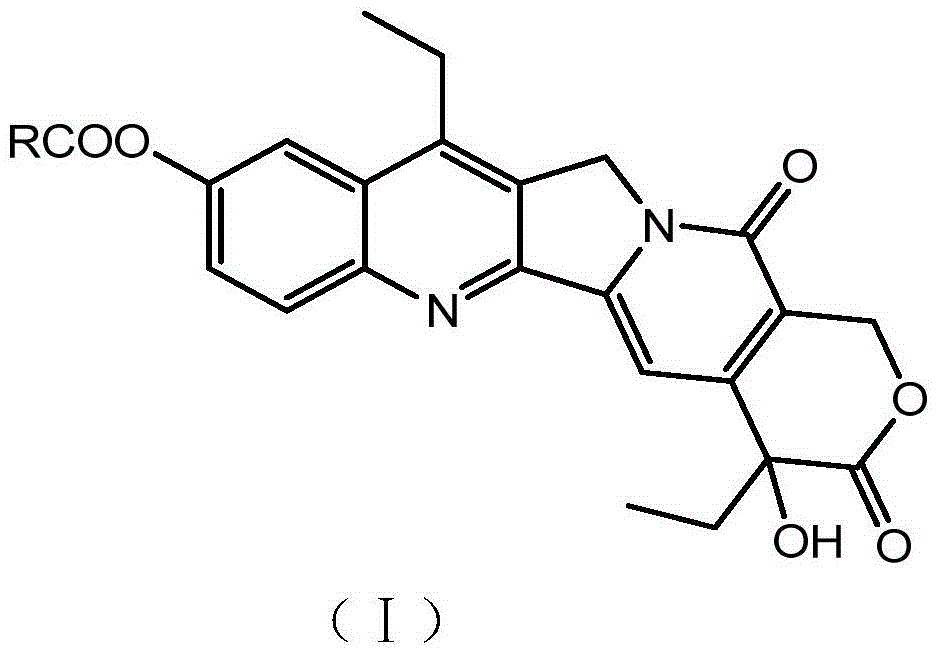
Saturated long-chain fatty acid-modified 7-ethyl-10-hydroxycamptothecin compound and long-circulating liposome thereof
A long-circulating liposome and long-chain fatty acid technology, applied in the field of medicine, can solve the problems of easy oxidation of unsaturated fatty acids, no in-depth research, no explanation, etc., to achieve stable properties, improve tumor distribution, and facilitate storage Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0061] Embodiment 1: Preparation of 7-ethyl-10-hydroxycamptothecin-10-palmitate
[0062]Add 1mmol of 7-ethyl-10-hydroxycamptothecin to the reaction vessel, add 0.2L of anhydrous chloroform to dissolve, then add 1.2mmol of DIPEA, stir for 5 minutes under ice bath conditions, slowly drop 1.2mmol Palmitoyl chloride, continue to react for 15 minutes under ice-bath conditions, and then react for 4 hours at room temperature. The reactant is recrystallized in acetone, and 620.12 mg of 7-ethyl-10-hydroxycamptothecin-10-palmitate is isolated , yield 98.37%.
[0063] The HNMR and MS (ESI) data of this material are as follows:
[0064] 1H NMR (CDCl3, 600MHz) δ: 8.22 (1H, d, J = 9.12Hz), 7.81 (1H, d, J = 2.46Hz), 7.64 (1H, s), 7.54 (1H, dd, J = 9.12and J = 2.46Hz), 5.74(1H, d), 5.30(1H, d), 5.25(2H, s), 3.86(1H, s), 3.15(2H, q), 2.65(2H, t), 1.95-1.85( 2H, m), 1.84-1.79 (2H, m), 1.50-1.26 (27H, m), 1.03 (3H, t), 0.87 (3H, t).
[0065] MS (ESI - )m / z:629(M-H + ).
[0066] The chemic...
Embodiment 2
[0068] Embodiment 2: Preparation of 7-ethyl-10-hydroxycamptothecin-10-palmitate
[0069] Add 1 mmol of 7-ethyl-10-hydroxycamptothecin to the reaction vessel, add 1 L of anhydrous dichloromethane to dissolve, then add 2 mmol of triethylamine, stir for 30 minutes under ice bath conditions, slowly add 2 mmol of Palmitoyl chloride, continue to react for 45 minutes under ice bath conditions, and then react for 24 hours at room temperature. The reactant is recrystallized in ethyl acetate, and 7-ethyl-10-hydroxycamptothecin-10-palmitate is isolated 621.33 mg, yield 98.56%.
Embodiment 3
[0070] Example 3: Preparation of 7-ethyl-10-hydroxycamptothecin-10-palmitate
[0071] Add 1mmol of 7-ethyl-10-hydroxycamptothecin to the reaction vessel, add 0.5L of anhydrous N,N-dimethylformamide to dissolve, then add 1.5mmol of DMAP, and stir for 15 minutes under ice bath conditions , slowly add 1.5 mmol of palmitoyl chloride dropwise, continue to react for 20 minutes under ice-bath conditions, and then react for 8 hours at room temperature. The reactant is recrystallized in cyclohexane, and 7-ethyl-10-hydroxy camptotheca Base-10-palmitate 610.28 mg, yield 96.81%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com