Application of mesenchymal stem cell in preparation of medicine used for treating M5 type leukaemia
A kind of mesenchymal stem cell and leukemia technology, applied in the field of medicine and biology, can solve the problems of poor prognosis, patients' intolerance, easy relapse, etc., achieve the effect of improving the ability of apoptosis, avoiding toxic and side effects, and meeting clinical needs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Isolation and subculture of umbilical cord mesenchymal stem cells
[0037] 1) Take the umbilical cord tissue of a newborn baby, put the umbilical cord in a petri dish and clean it with PBS containing 0.1% double antibody in the ultra-clean workbench, then put it into α-MEM medium, and cut the umbilical cord along the outer wall , strip three blood vessels, and cut the Wharton's jelly and umbilical cord wall into 1-2mm 3 tissue blocks, waiting to be digested;
[0038] 2) Prepare tissue-digesting enzymes (type II collagenase 250U / ml, dispase 100U / ml, hyaluronidase 10U / ml), dissolve in α-MEM medium at 37°C, filter and sterilize with a 0.22μm filter spare;
[0039] 3) Mix the shredded tissue pieces in 1) and the tissue-digesting enzyme solution prepared in 2) in a 1:1 volume ratio in a 50ml centrifuge tube, place in a shaker at 37°C, vibrate at 200rpm, and digest for about 3 hours. Wait until the tissue block is basically completely digested;
[0040] 4) The ...
Embodiment 2
[0043] Example 2: Identification of umbilical cord mesenchymal stem cells
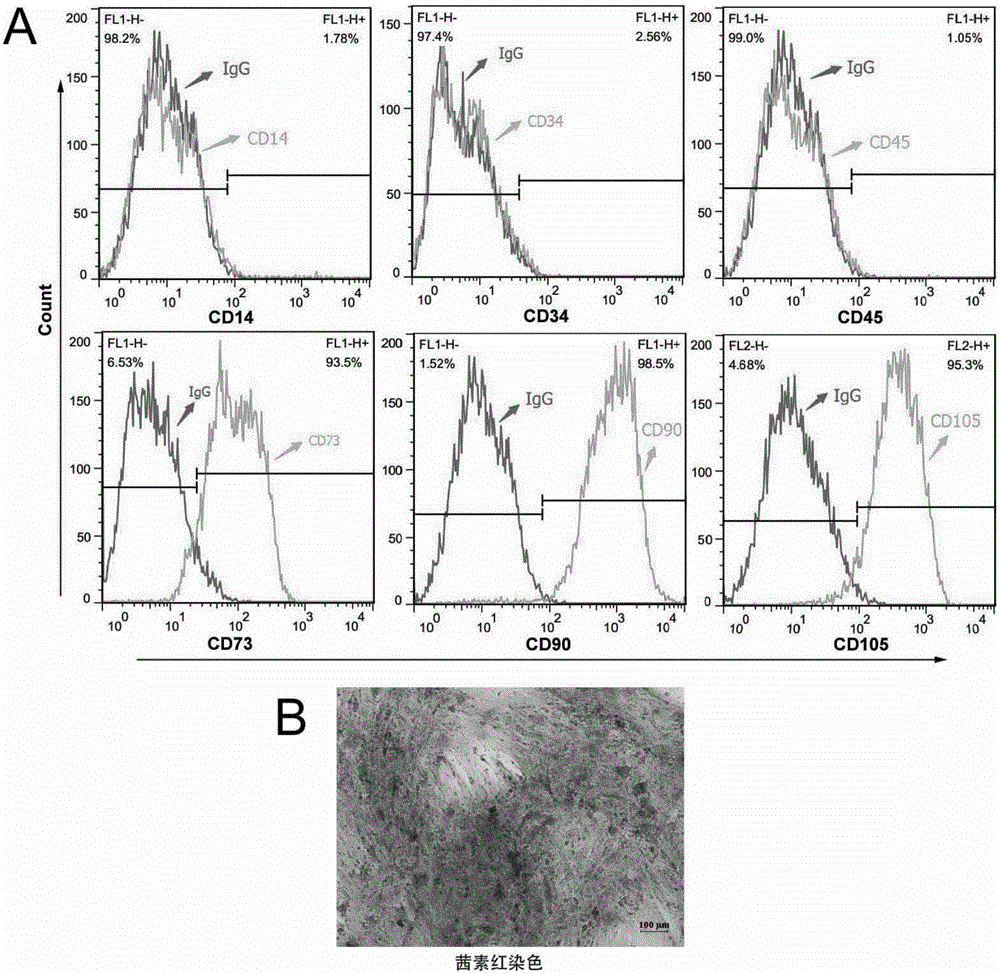
[0044] 1) Identification of MSCs cell surface markers by flow cytometry: Take MSCs grown to 90% confluence at passages P4-P6, digest with cell digestion solution, resuspend and centrifuge, and resuspend the cell pellet with PBS to 1-2×10 6 / ml of single cell suspension, 0.1ml in each flow tube; add fluorescently labeled antibodies CD14-FITC, CD34-FITC, CD45-FITC, CD73-FITC, CD90-FITC, CD105-FITC, IgG1, κ-FITC, IgG2α, κ-FITC, incubated on ice in the dark for 40 minutes; PBS was washed to remove unbound antibodies, then resuspended in 500ulPBS, and tested on the machine; CD14-FITC used IgG2α, κ-FITC as the isotype control, and the rest IgG1, κ-FITC were used as the isotype control; the identification results can be found in figure 1 , the expression of markers CD73, CD90, and CD105 is above 90%, and the expression of CD14, CD34, and CD45 is below 5%;
[0045] 2) MSCs osteogenic differentiation identifi...
Embodiment 3
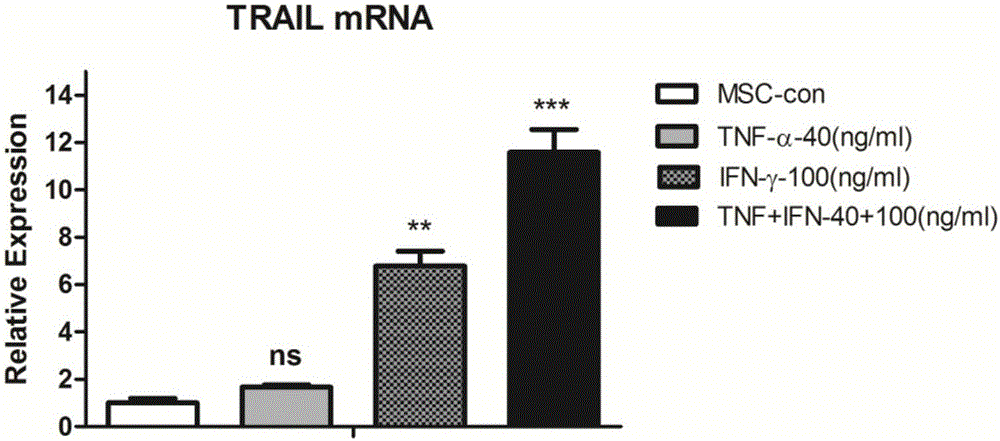
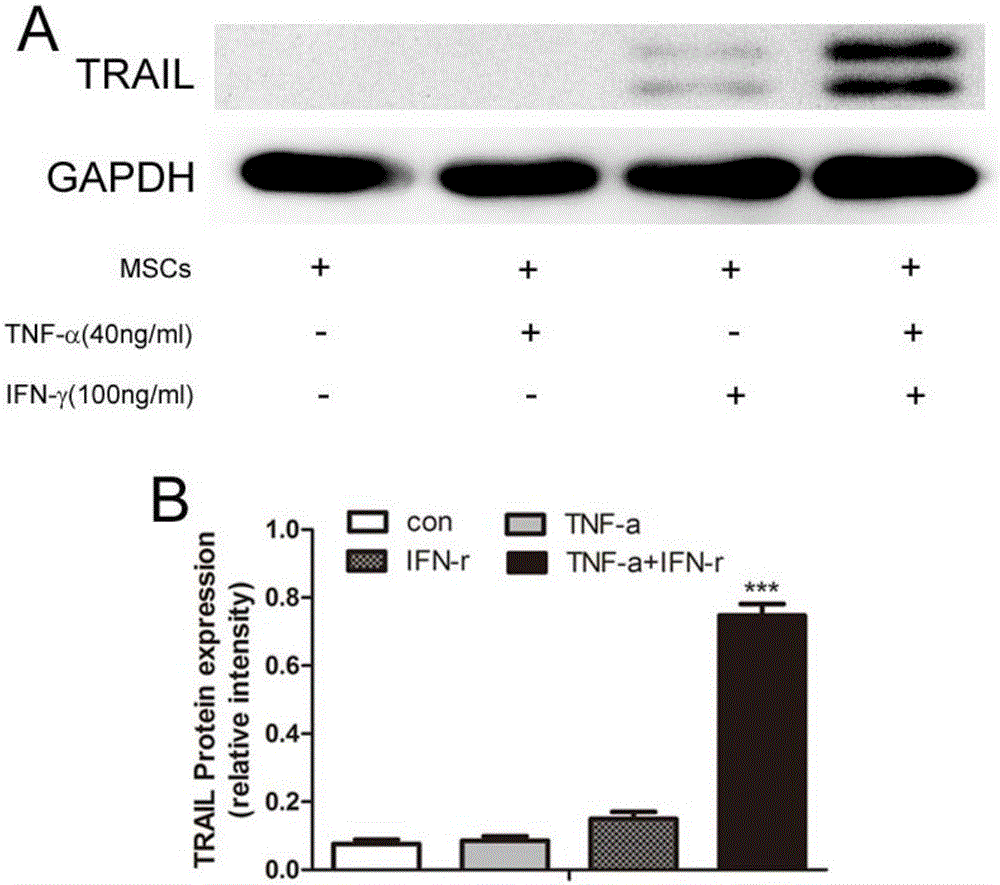
[0046] Embodiment 3: Real-timequantitativePCR experiment detects the expression of TRAIL
[0047] 1) Take the MSCs grown to 90% fusion in the P4-P6 generation, digest the cells, and re-inoculate into a 6-well cell culture plate, 2×10 5 / per well, 2ml of α-MEM medium containing 10% fetal bovine serum and 1% double antibody per well, placed at 37°C, 5% CO 2 1. After adherent culture in a saturated humidity incubator for 12 hours, inflammatory factor stimulation was added, and 4 groups were set up, TNF-α stimulation group alone, IFN-γ stimulation group alone, TNF-α and IFN-γ combined stimulation group , and an unstimulated control group, wherein the final concentrations of TNF-α and IFN-γ were 40ng / ml and 100ng / ml, respectively;
[0048] 2) After being stimulated by inflammatory factors for 24 hours, remove the medium, add 1ml Trizol to each well of the 6-well plate, pipette repeatedly, lyse the cells for 5min, and transfer the Trizol solution to 1.5ml RNasefree EP tube;
[004...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com