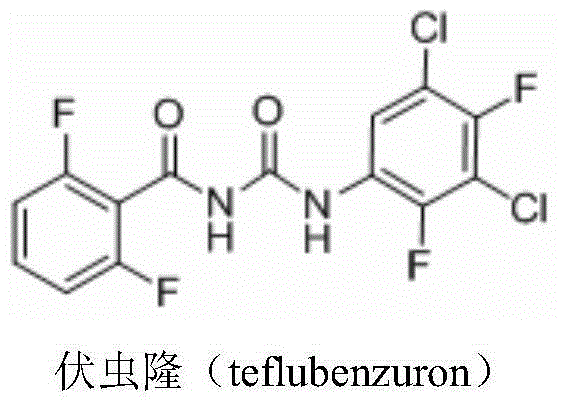
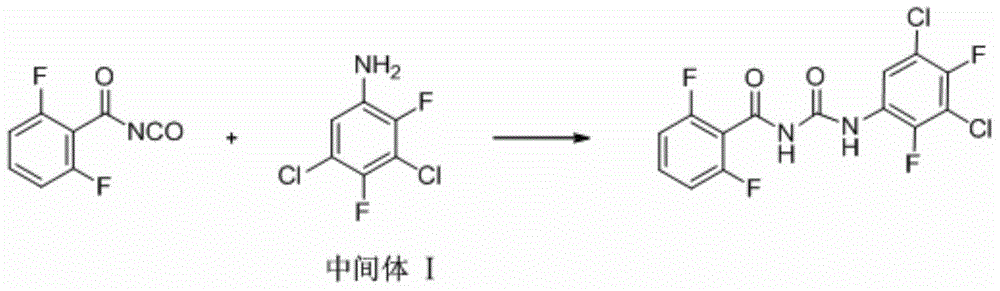
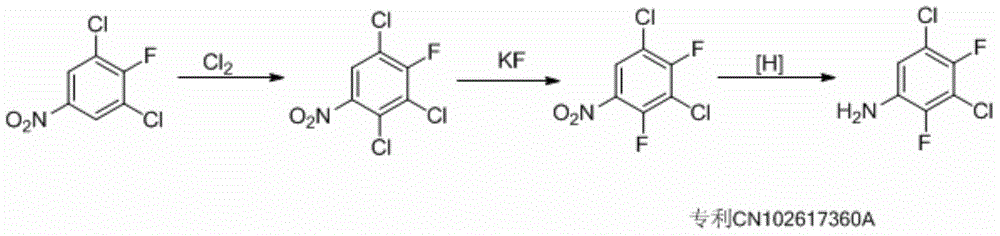
Preparation method for 3,5,-dichloro-2,4,-difluoroaniline
A technology of difluoroaniline and trichloroaniline, which is applied in the field of preparation of 3,5-dichloro-2,4-difluoroaniline, can solve the problems of insufficient output and low quality, and achieve the goal of less side reactions and higher yield. Good efficiency and quality, energy-saving effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0099] Preparation of intermediate 2,3,4-trichloronitrobenzene (II)
[0100] Preparation of mixed acid: Put 80g of nitric acid into a 500ml three-necked bottle, cool down to about 0°C, add 240g of concentrated sulfuric acid dropwise, and control the temperature not to exceed 10°C.
[0101]Add 200g of 1,2,3-trichlorobenzene (1.10mol) and 200g of dichloroethane into a 1000ml four-necked bottle, and add the mixed acid dropwise at a temperature of 50-60°C for 2 hours, and keep it warm for 30 minutes. Sampling GC analysis: 1,2,3-trichlorobenzene is less than 1%. Separate the layers: the organic layer was separated and the acid layer was extracted twice with dichloroethane. Combine the organic layers, wash with saturated sodium bicarbonate to pH = 7-8, dry over anhydrous sodium sulfate, filter, evaporate to dryness to recover dichloroethane, add 700g of n-hexane, heat up to dissolve, then cool down to about 15°C, pump After filtration and drying, 216g of finished product was obtai...
Embodiment 2
[0103] The preparation of intermediate 2,3,4-trichloroaniline (III):
[0104] Put 8g of iron powder (0.14mol), 80g of dichloroethane, 100g of water and 2g of ammonium chloride into a 500ml four-necked bottle. Under reflux, 11.5 g (0.051 mol) of 2,3,4-trichloronitrobenzene and 20 g of dichloroethane solution were added dropwise. After the dropwise addition, the solution was kept for 2 hours, and analyzed by GC, the reaction was basically completed. Filtrate, extract and wash the filtrate with water, separate the layers and combine the organic phases, and evaporate the solvent to obtain 8.2 g of the finished product, with a yield of 83%. 1H-NMR (400MHz, MeOD) δppm: 7.28 (d, 1H, J = 8Hz), 7.55 (d, 1H, J = 8Hz).
Embodiment 3
[0106] The preparation of intermediate 1,2,3,4-tetrachlorobenzene (IV):
[0107] Put 10 g (0.051 mol) of 2,3,4-trichloroaniline and 21 g of concentrated hydrochloric acid into a 500 ml four-necked bottle. Control the temperature in the range of -5-5°C and add dropwise an aqueous solution of 4g of sodium nitrite. After the addition is complete, keep the temperature for 1 hour for later use.
[0108] Put 50g of concentrated hydrochloric acid and 6g of cuprous chloride into another 1000ml four-neck bottle, add the previous nitrosation solution dropwise at about 50°C, raise the temperature to 80°C after adding, keep it warm for 1 hour, and then cool to 30 °C, extract the layers with dichloroethane, and evaporate to dryness to obtain 9 g of 1,2,3,4-tetrachlorobenzene. The GC analysis is over 98%, and the yield is 82%. 1H-NMR (400MHz, MeOD) δppm: 7.90 (s, 2H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com