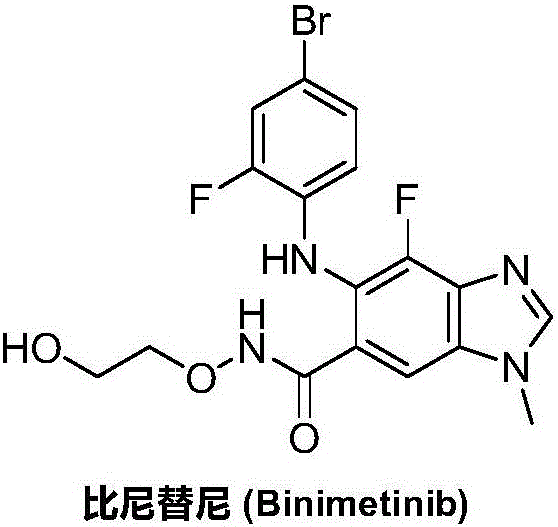
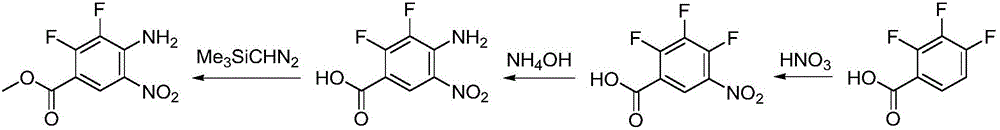
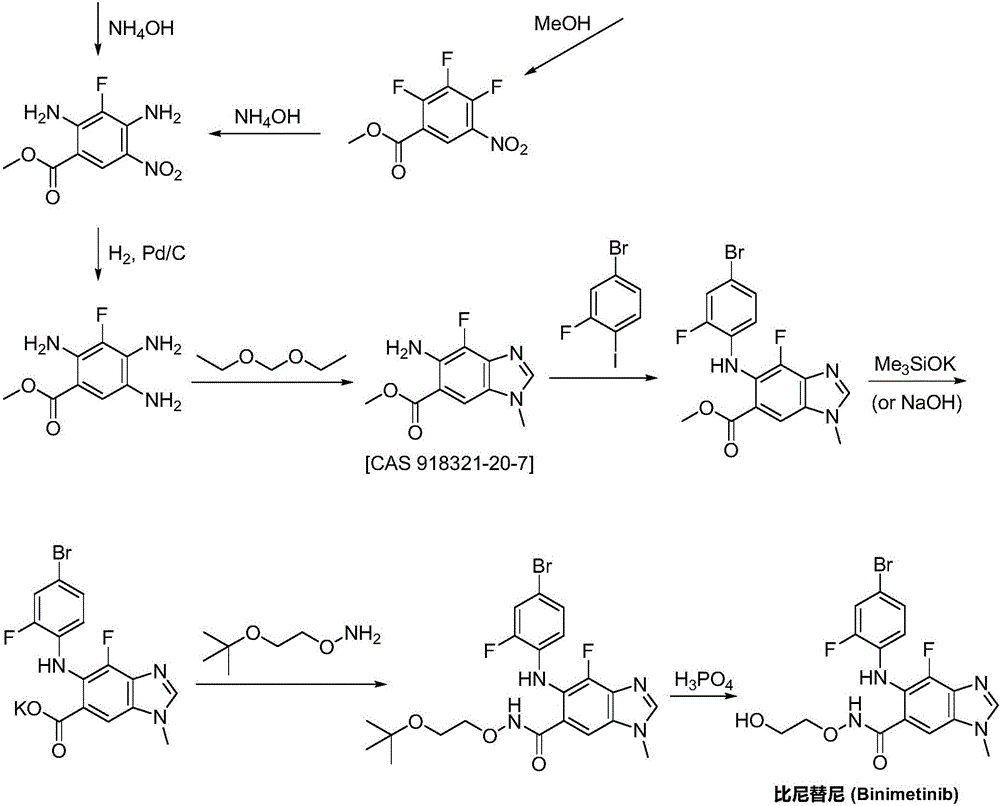
Synthesizing method for binimetinib
A technology of binitinib and synthesis method, applied in the direction of organic chemistry, etc., can solve problems such as unfavorable scale-up production and industrialization promotion, harsh reaction conditions and process, complicated operation, etc., and achieves favorable purification and purity, high yield , the effect of simple operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] A) Preparation of N-(2-tert-butoxyethoxy)-2,3,4-trifluoro-5-nitrobenzamide:
[0034]2,3,4-Trifluoro-5-nitrobenzoic acid (25.0g, 0.11mol) and N,N'-carbonyldiimidazole (22.9g, 0.14mol) were dissolved in tetrahydrofuran (110mL), stirred, and O- (2-tert-butoxyethyl)hydroxylamine (18.1g, 0.14mol), N,N-diisopropylethylamine (58.5g, 0.45mol) was added dropwise, and the reaction mixture was stirred at 50°C for 8h, followed by TLC spotting After confirming that the reaction is complete, the reaction solution is concentrated to dryness by rotary evaporation, adjusted to neutrality by adding dilute hydrochloric acid, extracted by adding ethyl acetate, dried over magnesium sulfate, concentrated by rotary evaporation to dryness, and recrystallized from methanol to obtain N-(2-tert-butoxy Ethoxy)-2,3,4-trifluoro-5-nitrobenzamide, off-white solid (33.5g), yield 88.0%, the reaction formula of this step is as follows:
[0035]
[0036] B) Preparation of N-(2-tert-butoxyethoxy)-2,4-d...
Embodiment 2
[0049] A) Preparation of N-(2-tert-butoxyethoxy)-2,3,4-trifluoro-5-nitrobenzamide:
[0050] 2,3,4-trifluoro-5-nitrobenzoic acid (30.0g, 0.14mol) and 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (27.4g, 0.18mol ) was dissolved in chloroform (240mL), stirred, O-(2-tert-butoxyethyl) hydroxylamine (23.5g, 0.18mol) was added, triethylamine (61.8g, 0.61mol) was added dropwise, and the reaction mixture was stirred at 55°C After reacting for 9 hours, TLC spotting confirmed that the reaction was complete, the reaction solution was concentrated to dryness by rotary evaporation, adjusted to neutrality by adding dilute hydrochloric acid, extracted by adding ethyl acetate, dried by magnesium sulfate, concentrated by rotary evaporation to dryness, and recrystallized from methanol to obtain N-( 2-tert-butoxyethoxy)-2,3,4-trifluoro-5-nitrobenzamide, off-white solid (39.9g), yield 87.4%, the reaction formula of this step is the same as that of Example 1 ;
[0051] B) Preparation of N-(2-ter...
Embodiment 3
[0060] A) Preparation of N-(2-tert-butoxyethoxy)-2,3,4-trifluoro-5-nitrobenzamide:
[0061] 2,3,4-trifluoro-5-nitrobenzoic acid (32.0g, 0.14mol) and 1-hydroxybenzotriazole (27.4g, 0.20mol) were dissolved in acetonitrile (150mL), stirred, and O- (2-tert-butoxyethyl)hydroxylamine (24.1g, 0.18mol), 4-dimethylaminopyridine (67.2g, 0.55mol) was added dropwise, and the reaction mixture was stirred at 60°C for 10h, and TLC was spotted to confirm that the reaction was complete. The reaction solution was concentrated to dryness by rotary evaporation, adjusted to neutrality by adding dilute hydrochloric acid, extracted by adding ethyl acetate, dried over magnesium sulfate, concentrated by rotary evaporation to dryness, and recrystallized from methanol to obtain N-(2-tert-butoxyethoxy) -2,3,4-trifluoro-5-nitrobenzamide, off-white solid (41.8g), yield 85.8%, the reaction formula of this step is the same as that of Example 1;
[0062] B) Preparation of N-(2-tert-butoxyethoxy)-2,4-diamino-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com