Cyclo-octadiene complex containing dinitrogen ligand low-valent metal rhodium (I) and preparation method thereof
A technology of cyclooctadiene and nitrogen ligands, which is applied in the field of potential coupling and hydrogenation reaction catalysts and its synthesis, and can solve the problems of metal center oxidation, low yield, and cumbersome steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
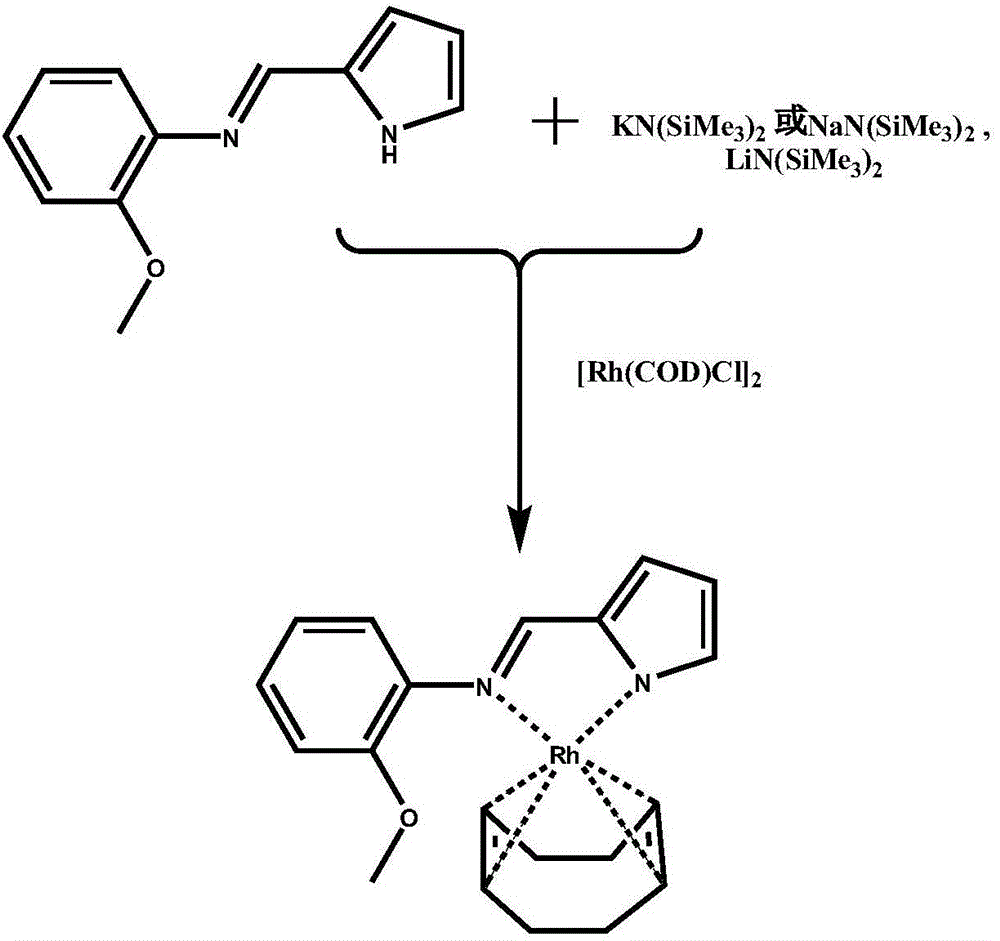
[0020] A cyclooctadiene complex containing dinitrogen ligand low-valent metal rhodium (I) and its preparation method, the reaction schematic diagram is as attached figure 1 As shown, the specific steps are as follows:
[0021] (1) React a certain proportion of o-methoxyaniline and pyrrole-2-carboxaldehyde in anhydrous alcoholic solvent, acetic acid is used as a catalyst, the reaction temperature is 70-90°C, and the reaction is stirred for 8-12 hours to obtain diazepam Phosphine ligand 2-(pyrrole-2-methyleneamino) anisole;
[0022] (2) Under the protection of an inert gas, 2-(pyrrole-2-methyleneamino) anisole and bis(trimethylsilyl)amino strong base are stirred in anhydrous solvent at room temperature for deprotonation reaction, and the reaction time for 20-40 minutes, and then reacted with (1,5-cyclooctadiene) rhodium (I) dimer compound in situ for 3-6 hours to obtain a mixture that was easily evaporated to dryness, and its solvent was evaporated After drying, use an organic...
Embodiment 1
[0030] Synthesis of the cyclooctadiene complex of dinitrogen ligand metal rhodium (I), the anhydrous solvent used in the step (2) is anhydrous tetrahydrofuran, the base used is two (trimethylsilyl) amide lithium, deprotonation reaction The reaction temperature between the product and (1,5-cyclooctadiene)rhodium(I) chloride dimer is 45° C., and the reaction time is 6 hours.
[0031] (1) Weigh 950mg (10mmol) of pyrrole-2-carbaldehyde and add it to a 100ml round-bottomed flask with a magnetic stirrer, measure 20ml of anhydrous methanol with a syringe and add it to the reaction flask, add a drop of acetic acid, and keep stirring in batches 1.23 g (10 mmol) of o-methoxyaniline was added, and the reaction system was kept under reflux for 8 hours, and the resulting mixture was cooled to obtain the solid product 2-(pyrrole-2-methyleneamino)anisole.
[0032] (2) The reaction is carried out under nitrogen protection conditions by utilizing a double row tube device. Weigh 190 mg of 2-(p...
Embodiment 2
[0034] The synthesis of the cyclooctadiene complex of dinitrogen ligand metal rhodium (I), the anhydrous solvent anhydrous tetrahydrofuran used in the step (2), the alkali used is two (trimethylsilyl) sodium amide, and the deprotonation reaction product The temperature of the reaction with (1,5-cyclooctadiene)rhodium(I) chloride dimer is 45° C., and the reaction time is 4 hours.
[0035] (1) For the preparation of 2-(pyrrole-2-methyleneamino)anisole, see step 1 of Example 1.
[0036] (2) The reaction is carried out under nitrogen protection conditions by utilizing a double row tube device. Weigh 190 mg of 2-(pyrrole-2-methyleneamino) anisole (0.95 mmol, dissolved in 8 ml of anhydrous tetrahydrofuran solvent), then pour it into a 25 ml round-bottomed flask with a magnetic stirring bar, add 1 ml of bis(tri A tetrahydrofuran solution of methylsilyl) sodium amide (1.0mmol), reacted for 40 minutes to obtain a yellow solution; weigh (1,5-cyclooctadiene) rhodium chloride (I) dimer c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com