Legionella pneumophilia antigen near-infrared fluorescent detection kit and purpose thereof
A technology for Legionella pneumophila and fluorescence detection is applied in the field of near-infrared fluorescence detection kits for Legionella pneumophila antigens, which can solve the problem that the detection technology level of Legionella pneumophila is lagging behind, the antibody content cannot reach the detection level, and it is not suitable for clinical detection. application and other issues, to achieve high sensitivity, good specificity, and good specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] 1. Sputum collection and storage
[0055] Under the guidance of medical staff, the patient coughs up the sputum in the deep part of the respiratory tract forcefully, spits it into a sterile jar, and immediately closes the lid tightly and saves it for later use. The samples to be processed should be stored in a refrigerator at 4°C.
[0056] 2. Urine collection and storage
[0057] Under the guidance of medical staff, the patient washes and collects the first urine in the morning, and the collected urine is stored in a clean and sterile container for future use. Urine samples can be stored at 2-8°C for 72 hours. If they need to be stored for a longer period of time, the samples must be stored at -20°C. Repeated freezing and thawing of samples should be avoided, otherwise erroneous experimental results will be produced. Samples should not be stored in an automatic defrost refrigerator.
[0058] 3. Antigen preparation:
[0059] Purchase Legionella pneumophila strains fr...
Embodiment 2
[0074] Comparison experiment of Legionella pneumophila antigen near-infrared fluorescence detection method and sputum bacterial culture:
[0075] Sputum bacterial culture:
[0076] The sputum was pretreated (the method of combining 1ug / ml trypsin solution with glass fragment shaking was used to pretreat the sputum before inoculation and culture), and the pretreated samples were immediately inoculated on the GVPC selective medium. Gram-stained colonies of suspected Legionella pneumophila were inoculated on BCYE-a and BCYE-Cys agar plates with negative Gram-stained colonies, and cultured in a 37°C incubator for 2 days. Any colony that grows on BCYE-a medium but does not grow on BCYE-Cys medium can be considered as Legionella pneumophila.
[0077] Kit detection:
[0078] The sample to be tested is a sputum dilution, which is diluted with 0.01M pH7.2 PBS buffer, and the dilution ratio is 1:1.
[0079] The sample to be tested and the kit are equilibrated to room temperature to s...
Embodiment 3
[0088] Cross experiment:
[0089] Add the prepared Enterococcus faecalis, Enterococcus faecium, Escherichia coli, Acinetobacter baumannii, Klebsiella, Lactobacillus, Neisseria gonorrhoeae, green Pseudomonas pyogenes, Escherichia coli, Mycoplasma hominis, Ureaplasma urealyticum, Staphylococcus aureus, Streptococcus and Helicobacter pylori (1×10 6 CFU / ml), to carry out the cross experiment detection to detect whether the above bacteria have an impact on the test results of the kit. The results of the cross experiment between the Legionella pneumophila antigen near-infrared fluorescence detection reagent and the bacteria are shown in Table 2. There was no cross experiment in each bacteria cross experiment. Cross-reactivity occurs:
[0090] Table 2
[0091] cross
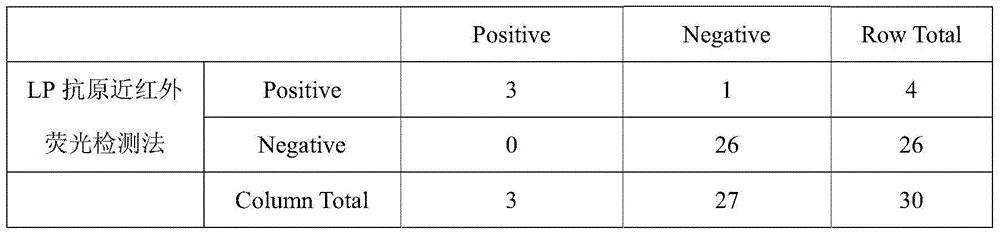
[0092] As can be seen from Table 1 in Example 2 and Table 2 in Example 3, in the detection of a total of 30 clinical samples, 4 cases were detected as LP positive by near-infrared fluorescence detection meth...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| Sensitivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com