Preparation method of 2-(methyl sulphonyl)-10H-phenothiazine
A technology of methylsulfonyl and phenothiazine, which is applied in the field of preparation of 2--10H-phenothiazine, can solve problems such as high cost, unfavorable industrialized production, etc., and achieves improved yield, environmental protection, and increased contact area effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
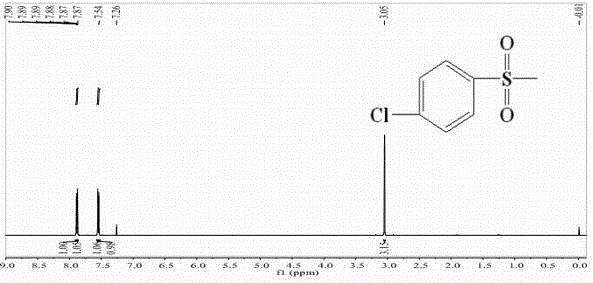
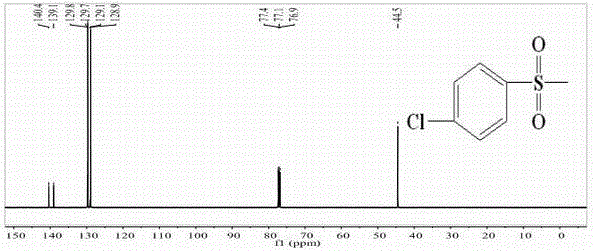
[0048] Example 1: Synthesis of p-chlorophenylsulfone (2a)
[0049]
[0050] In a 250ml three-neck round bottom flask equipped with a stirrer, dropping funnel, thermometer and tail gas recovery device, add 21.2g of sodium sulfite, 26.5g of sodium bicarbonate, and 100ml of water, raise the temperature to 75°C while stirring, and weigh 31.2 Add 4 g of p-chlorobenzenesulfonyl chloride to the three-necked flask in batches within 3 hours, keep warm and reduce for 1 hour, slowly add (35.9g sodium chloroacetate dissolved in 50ml water) aqueous solution of sodium chloroacetate dropwise through the constant pressure dropping funnel, and the temperature of the mixture is raised to 105 -107°C, reflux reaction for 15h, after the reaction, pour out the reactant, cool, filter with suction, pour the solid into a beaker, add an appropriate amount of CCl 4 Carry out purification, stand overnight and then suction filter, put into vacuum drying box and dry, obtain white needle-like crystal, we...
Embodiment 2
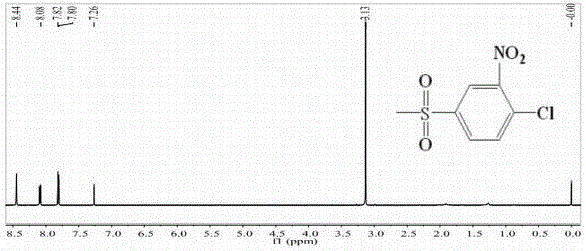
[0053] Example 2: Synthesis of 2-nitro-4-thiamphenicol chlorobenzene (2b)
[0054]
[0055] In a 100ml three-neck round bottom flask equipped with a stirring dropping funnel, a thermometer and a stirrer, add 24ml of concentrated sulfuric acid and 11.3g of p-chlorophenylsulfone, stir to dissolve p-chlorophenylsulfone, and slowly Add 6ml of nitric acid dropwise. After the dropwise addition, keep the temperature at 25°C and keep it warm for 2 hours. Then pour the reaction solution into a beaker, add an appropriate amount of ice water into the beaker while stirring, and a white precipitate will precipitate. Cool to room temperature and wash with suction. After deacidification and vacuum drying, a white powdery solid was obtained, which weighed 13.9 g. The melting range was 124.2-125.1°C as determined by the melting point apparatus. The purity was 99.9% and the yield was 99.7% as determined by the gas phase.
[0056] Each hydrogen atom belongs to: 1 H NMR (500M, CDCl 3 ) : δ:...
Embodiment 3
[0059] Example 3: Synthesis of Compound M1
[0060]
[0061] In a 250ml three-necked round-bottomed flask equipped with a stirrer, dropping funnel, and thermometer, add 23.56g of 3-nitro-p-chlorophenylmethyl sulfone (0.1mol) and 100ml of absolute ethanol in sequence, stir and heat up to 40°C. Dissolve completely, add dropwise a mixed solution of 34.4g 20% sodium ethoxide ethanol solution and 19.21 (0.101mol) g 2-bromothiophenol, and raise the temperature to 80°C at the same time, after about 0.5h dropwise addition, continue to reflux for 24h to stop the reaction, cool to At room temperature, recrystallize with absolute ethanol, collect the product by suction filtration, and dry to obtain 38.47 g of a yellow powder product. The melting range measured by the melting point instrument is 162.4~163.0°C, the purity is 99.9%, and the yield is 99.0%.
[0062] Each hydrogen atom is assigned to δ: 1 HNMR (500M, CDCl 3 ):8.82(s,1H),7.86(d,1H),7.83(d,1H),
[0063] 7.76 (d, 1H), 7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com