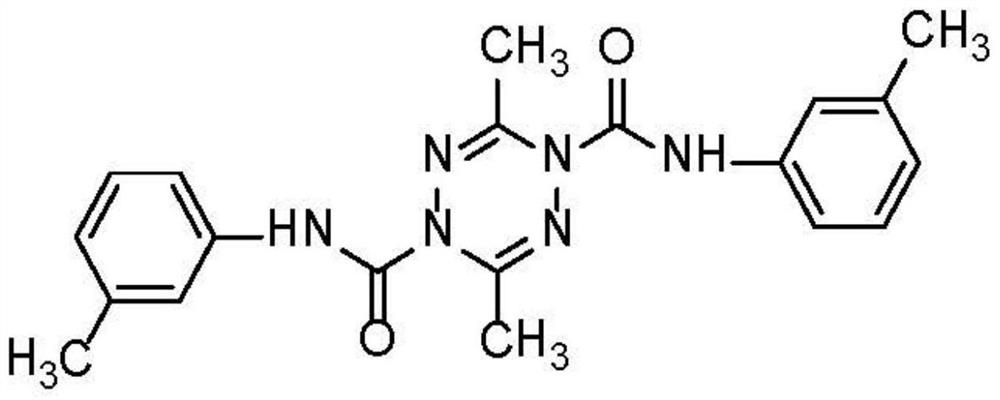
A tetrazinedicarboxamide liposome preparation and preparation method thereof
A technology of tetrazine dicarboxamide and liposome preparation, which is applied in the preparation of new tetrazine dicarboxamide formulations, tetrazine dicarboxamide liposome preparation and its preparation field, and can solve problems such as retention and inability to absorb drugs , to achieve the effect of low toxicity, beneficial to disease treatment, and beneficial to removal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Tetrazinedicarboxamide liposome preparation, this liposome comprises following composition and quality:
[0034] Tetrazine dicarboxamide: 4mg;
[0035] Cholesterol: 20mg;
[0036] Lecithin (PC content 98%): 100mg;
[0037] Ethanol: 10mL;
[0038] Phosphate buffer solution (pH 6.8): 20 mL.
[0039] Dissolve the prescribed amount of tetrazinedicarboxamide, lecithin, and cholesterol in ethanol, stir and dissolve to prepare solution A; put solution A in a flask, and use a rotary evaporator to remove the organic solvent ethanol, and the constant temperature water bath of the rotary evaporator is 45 °C, rotation speed 100r / min, vacuum degree greater than 0.09MPa, wait for a dry film to form on the bottle wall, and continue to evaporate until the solvent evaporates completely. Add the prescribed amount of phosphate buffered saline solution in the flask, and the probe is ultrasonicated for 10 minutes for hydration, and the solution is filtered with a 0.45um microporous memb...
Embodiment 2
[0042] Tetrazinedicarboxamide liposome preparation, this liposome comprises following composition and quality:
[0043] Tetrazine dicarboxamide: 4mg;
[0044] Cholesterol: 15mg;
[0045] Lecithin (PC content 98%): 150mg;
[0046] Vitamin E: 2mg;
[0047] Diethyl ether: 15mL;
[0048] Phosphate buffer solution (pH 6.4): 25 mL.
[0049]Dissolve the prescribed amount of tetrazinedicarboxamide, lecithin, cholesterol, and vitamin E in ether, stir and dissolve to prepare solution A; inject solution A into the continuously stirring phosphate buffer solution through the syringe, and obtain a milky white mixture solution, the mixed solution is placed in a 55°C constant temperature water bath, continuously stirred, the organic solvent is volatilized and removed, and then filtered with a 0.45um microporous membrane to obtain a slightly clear milky white drug liposome solution.
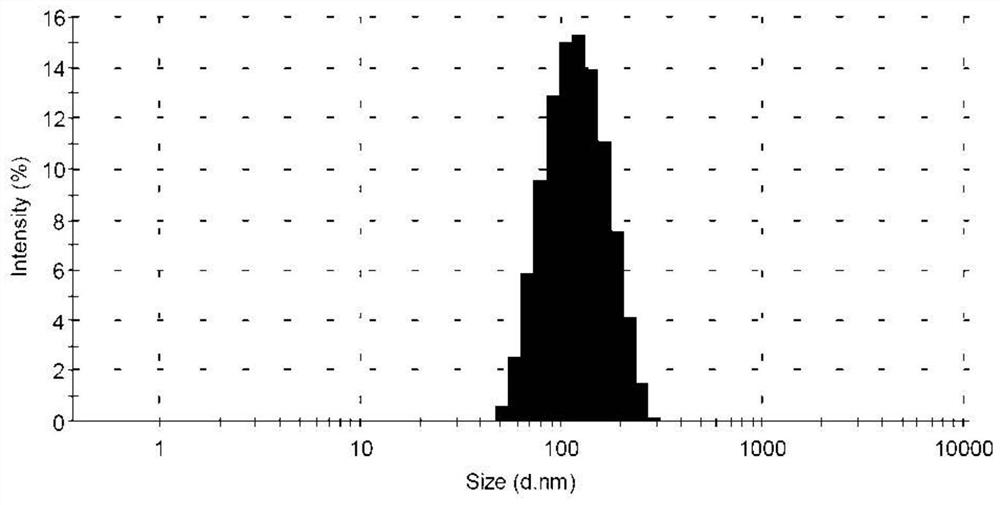
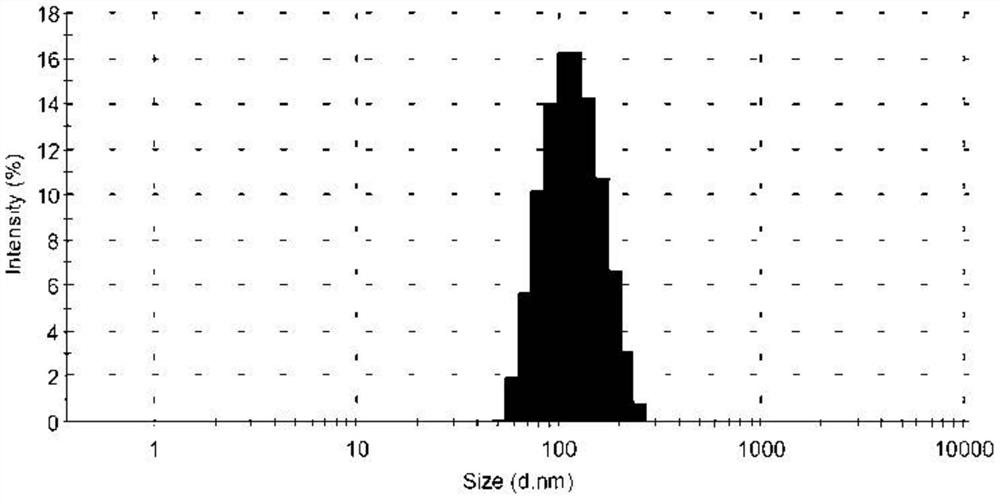
[0050] The obtained tetrazinedicarboxamide liposome solution has a particle diameter of 105.4 nm, a polyd...
Embodiment 3
[0052] Tetrazinedicarboxamide liposome preparation, this liposome comprises following composition and quality:
[0053] Tetrazine dicarboxamide: 8mg;
[0054] Cholesterol: 10mg;
[0055] Soy lecithin (PC content 70%): 80mg;
[0056] Vitamin E: 6mg;
[0057] Chloroform: 25mL;
[0058] Phosphate buffer solution (pH 7.0): 12 mL.
[0059] Dissolve the prescribed amount of tetrazinedicarboxamide, lecithin, cholesterol, and vitamin E in chloroform, and ultrasonically dissolve to prepare solution A; put solution A in a flask, use a rotary evaporator to remove the organic solvent ethanol, and use a rotary evaporator to keep the temperature constant The temperature of the water bath is 40°C, the rotation speed is 100r / min, and the vacuum degree is greater than 0.09MPa. After a dry film is formed on the bottle wall, continue to evaporate until the solvent evaporates completely. Add the prescribed amount of phosphate buffered saline solution in the flask, and the probe is ultrasonic...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com