Cefoperazone sodium and sulbactam sodium composition
A technology of cefoperazone sodium sulbactam sodium and cefoperazone sodium, which is applied in the field of medicine, can solve the problems of poor uniformity, fluidity and stability of raw materials, large dosage, and reduced content of active ingredients of medicines, and reduce the types, contents, and types of impurities And the effect of less dosage and reducing the accumulation in the body
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
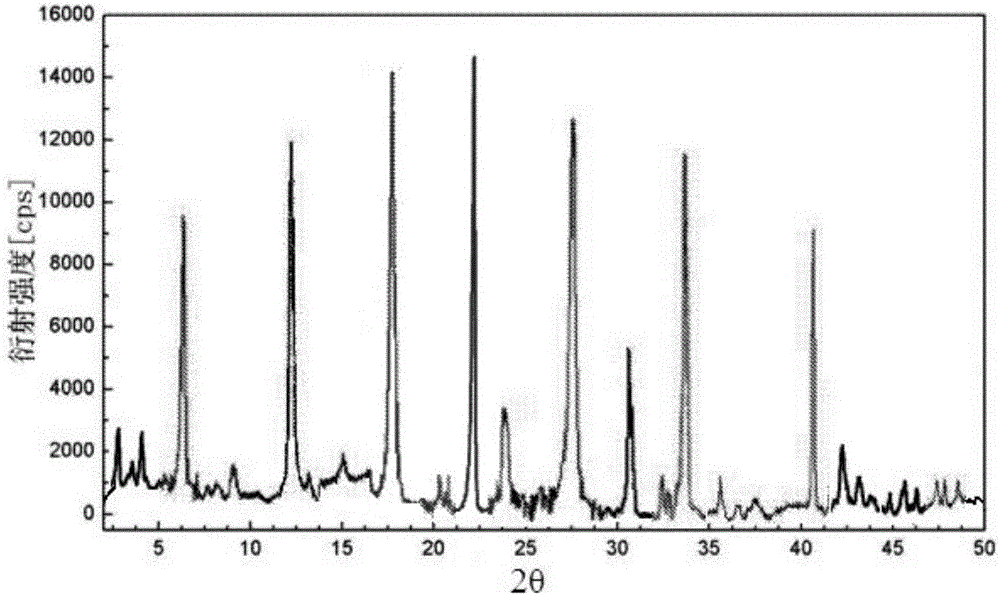
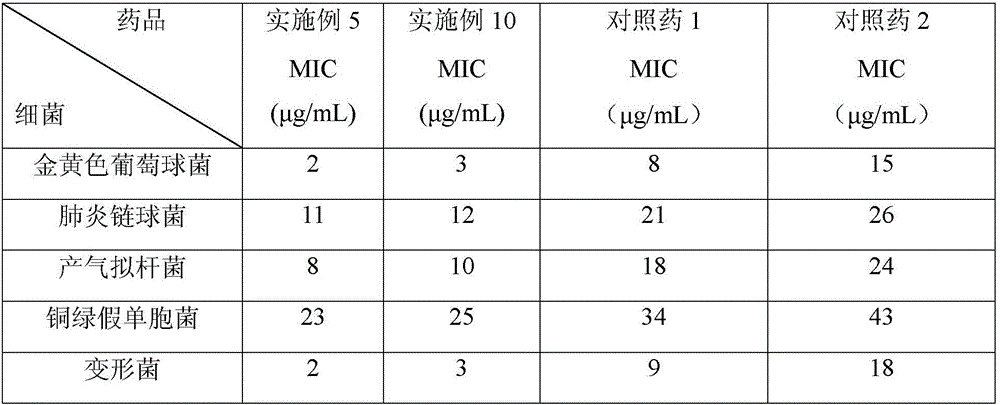
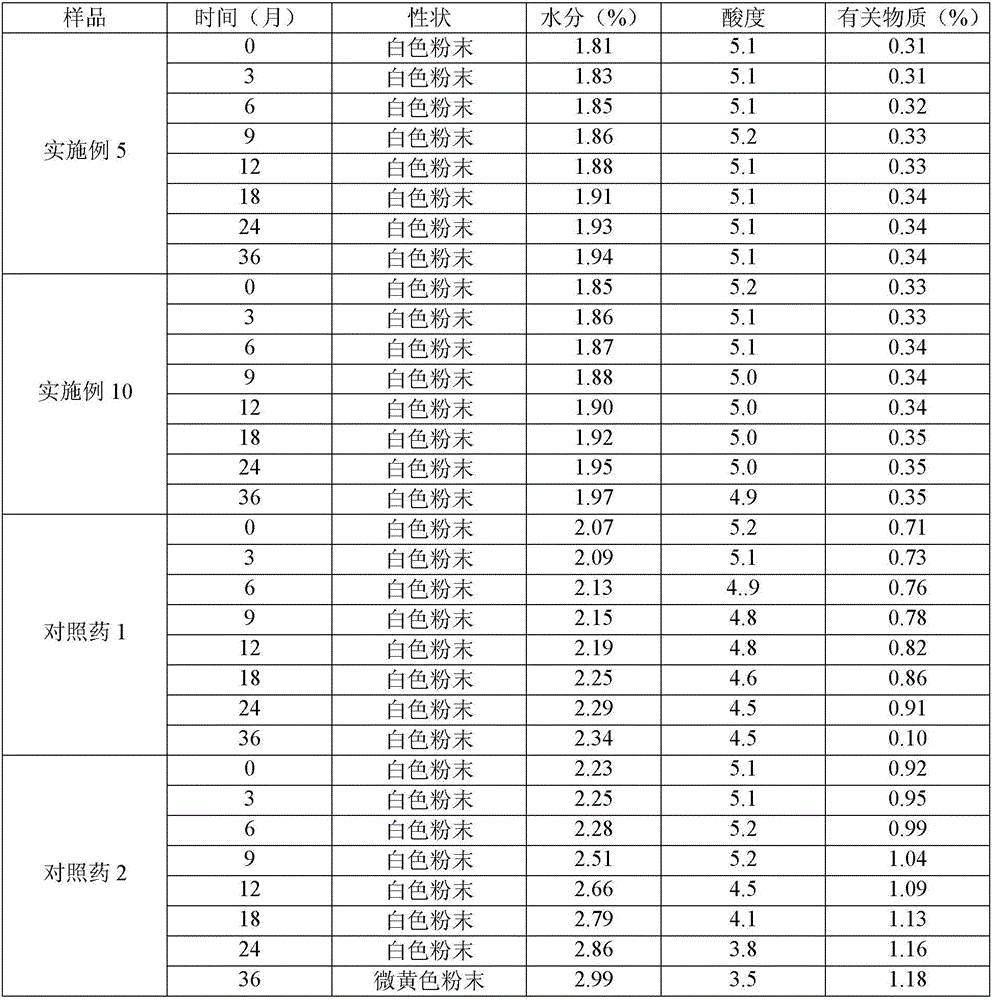
Image
Examples
Embodiment 1
[0036] The preparation of embodiment 1 sulbactam sodium crystal
[0037] (1) Grind the crude product of sulbactam sodium, cross a 100 mesh sieve, then add a mixed solution of deionized water and methanol whose volumetric amount is 18 times the weight of sulbactam sodium, and the volume ratio of deionized water and methanol is 8.5:1 , stirred evenly to dissolve, and adjusted the pH to 8;
[0038] (2) adding activated carbon for decolorization, filtering to obtain a clear solution;
[0039] (3) Add dropwise the mixed solution of acetone and ethyl acetate with a volume ratio of 1:3.5 to the above clear solution under the condition of stirring rate of 30rmp, then put it into a sound field with a frequency of 36KHz and an output power of 38W at a speed of 32rmp Stir under low temperature for 16 minutes to cool down to 5°C, let stand for 25min, then stir at 25rmp for 40min and cool to -18°C, stand for crystallization for 26h, wash, and vacuum dry to obtain the aztreonam crystal.
...
Embodiment 2
[0041] The preparation of embodiment 2 sulbactam sodium crystals
[0042] (1) Grind the crude product of sulbactam sodium, cross a 100-mesh sieve, then add a mixed solution of deionized water and methanol whose volumetric amount is 19 times the weight of sulbactam sodium, and the volume ratio of deionized water and methanol is 9:1 , stirred evenly to dissolve, and adjusted the pH to 8.5;
[0043] (2) adding activated carbon for decolorization, filtering to obtain a clear solution;
[0044] (3) Add dropwise the mixed solution of acetone and ethyl acetate with a volume ratio of 1:3.7 to the above clear solution at a stirring rate of 35rmp, then put it into a sound field with a frequency of 36KHz and an output power of 38W at a speed of 34rmp Stir under low temperature for 18 minutes and cool down to 7°C, stand still for 28 minutes, then stir at 27rmp for 44min and cool down to -16°C, stand for crystallization for 24h, wash, and dry in vacuum to obtain the aztreonam crystals.
...
Embodiment 3
[0046] The preparation of embodiment 3 sulbactam sodium crystals
[0047] (1) Grind the crude product of sulbactam sodium, cross a 100-mesh sieve, then add a mixed solution of deionized water and methanol whose volumetric amount is 20 times the weight of sulbactam sodium, and the volume ratio of deionized water and methanol is 9.5:1 , stirred evenly to dissolve, and adjusted the pH to 9;
[0048] (2) adding activated carbon for decolorization, filtering to obtain a clear solution;
[0049] (3) Add dropwise a mixed solution of acetone and ethyl acetate with a volume ratio of 1:4 to the above clear solution under the condition of a stirring rate of 36rmp, and then put it into a sound field with a frequency of 36KHz and an output power of 38W at a speed of 36rmp Stir under low temperature for 19 minutes and cool down to 8° C., stand still for 30 minutes, then stir at 28 rpm for 46 minutes and cool down to -15° C., stand for crystallization for 28 hours, wash, and dry in vacuum t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com