Conjugated polymer electron donor material of polymer solar cell blended active layer and preparation method of conjugated polymer electron donor material
A conjugated polymer, solar cell technology, applied in the direction of electric solid device, semiconductor/solid state device manufacturing, circuit, etc., to achieve the effect of improving processability, good solubility and solution processability, easy modification and solubilization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
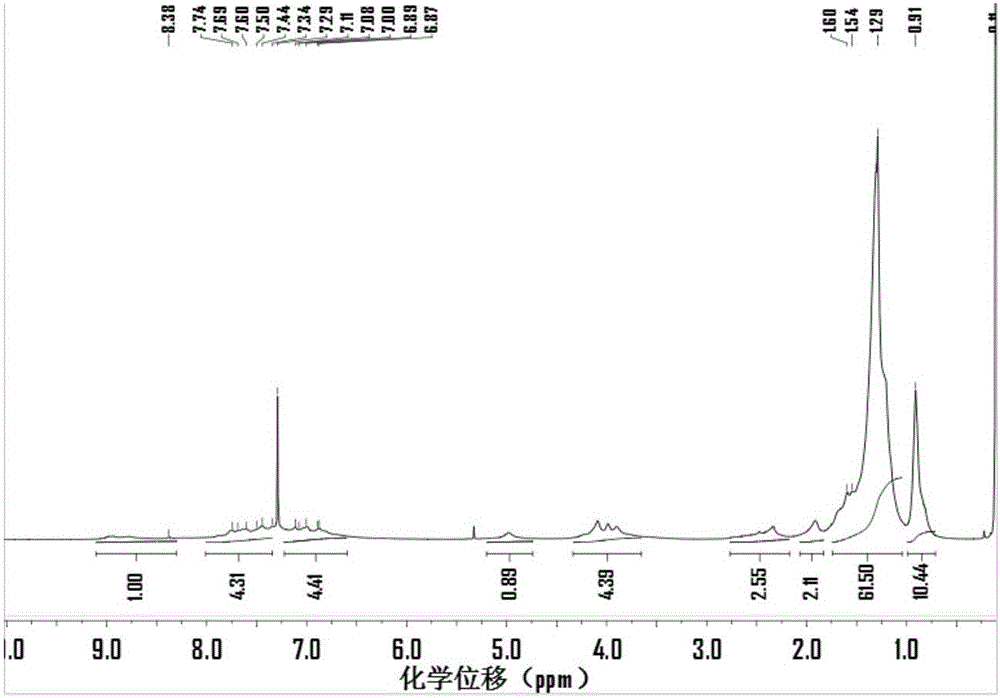
Examples
Embodiment 1
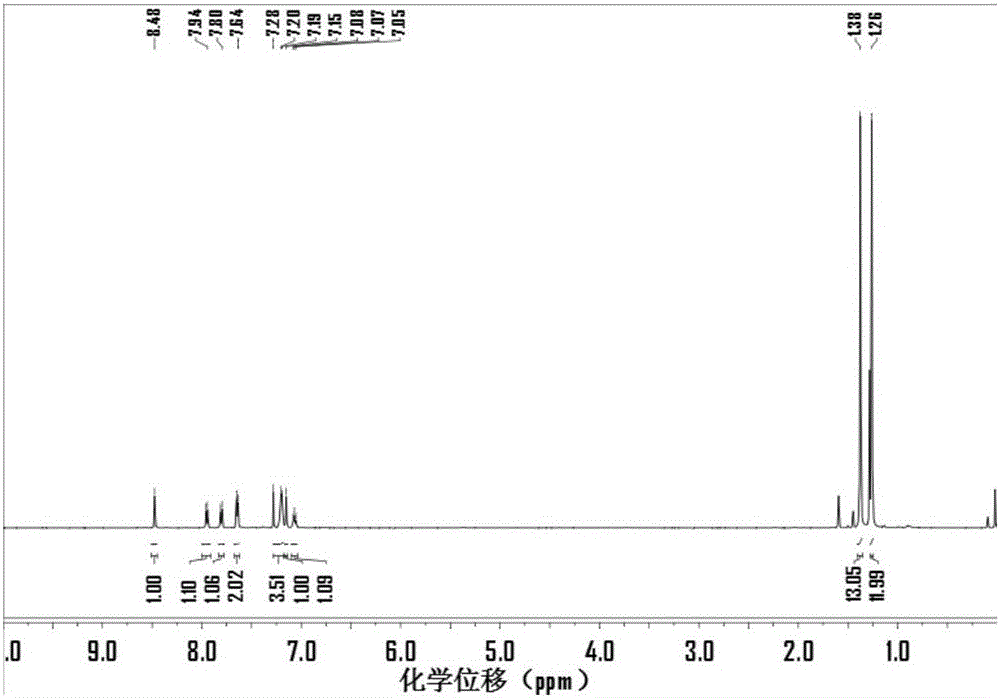
[0035] (1) 2,7-dibromo-fluoren-9-one
[0036]
[0037] The raw material fluorenone (2.5g, 14mmol) was added to 50mL of deionized water, and the aqueous solution was heated to 80°C and stirred for 1 hour, and bromine (4.4g, 27.8mmol) was slowly added dropwise. The molar ratio of fluorenone to bromine was 1:2. Then be warming up to 85 DEG C and react for 6 hours; stop the reaction, wait for the solution to cool to room temperature, pour the reaction mixture into deionized water, add aqueous sodium hydroxide solution (10%, wt%), filter the yellow precipitate generated, and simultaneously use a large amount of Washed with deionized water and dried in vacuo to obtain a yellow solid product with a yield of 90%.
[0038] (2) 2,7-dibromo-N-(4-fluoro)phenyl-imine-9H-fluorene
[0039]
[0040] Dissolve 4-fluoroaniline (1.0g, 9.3mmol) and triethylamine (3.6g, 36mmol) in 65mL of anhydrous tetrahydrofuran, lower the temperature to -15°C under the protection of argon and stir for 30 ...
Embodiment 2
[0049] (1) 2,7-dibromo-fluoren-9-one
[0050]
[0051] The raw material fluorenone (2.5g, 14mmol) was added to 50mL of deionized water, and the aqueous solution was heated to 80°C and stirred for 1 hour, and bromine (6.6g, 42mmol) was slowly added dropwise. The molar ratio of fluorenone to bromine was 1:3, and then The temperature was raised to 90° C. for 10 hours; the reaction was stopped, and the solution was cooled to room temperature, the reaction mixture was poured into deionized water, an aqueous sodium hydroxide solution (10%, wt%) was added, and the resulting yellow precipitate was filtered, while a large amount of Washing with deionized water and vacuum drying gave a yellow solid product with a yield of 91%.
[0052] (2) 2,7-dibromo-N-(4-methyl)phenyl-imine-9H-fluorene
[0053]
[0054] Dissolve 4-methyl-aniline (1.1g, 10mmol) and triethylamine (4.5g, 44mmol) in 65mL of anhydrous tetrahydrofuran, lower the temperature to -25°C and stir for 30 minutes under the ...
Embodiment 3
[0063] (1) 2,7-dibromo-fluoren-9-one
[0064]
[0065] The raw material fluorenone (2.5g, 14mmol) was added to 50mL of deionized water, the aqueous solution was heated to 80°C and stirred for 1 hour, and bromine (11g, 69mmol) was slowly added dropwise, the molar ratio of fluorenone to bromine was 1:5, and then the temperature was raised Reaction at 95° C. for 12 hours; stop the reaction, wait for the solution to cool to room temperature, pour the reaction mixture into deionized water, add aqueous sodium hydroxide solution (10%, wt%), filter the yellow precipitate generated, and simultaneously use a large amount of deionized Washed with deionized water and dried under vacuum to obtain a yellow solid product with a yield of 89%.
[0066] (2) 2,7-dibromo-N-naphthyl-imine-9H-fluorene
[0067]
[0068] 2-Naphthylamine (1.6g, 11mmol) and triethylamine (5.4g, 53mmol) were dissolved in 65mL of anhydrous tetrahydrofuran, under the protection of argon, the temperature was lowered...
PUM
| Property | Measurement | Unit |
|---|---|---|
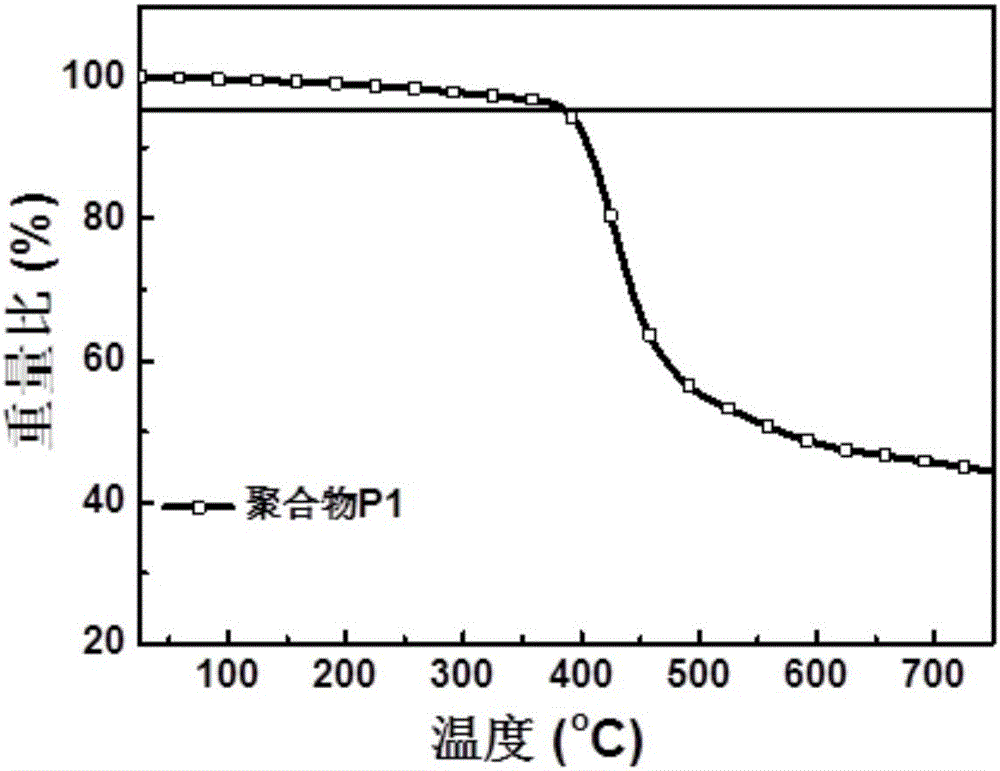
| Thermal decomposition temperature | aaaaa | aaaaa |
| Optical bandgap | aaaaa | aaaaa |
| Thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com