Cu<2+> chemical sensor, and preparation method and application thereof
A chemical sensor and nanomaterial technology, applied in the field of preparation of Cu2+ chemical sensor, can solve the problems of large background interference, complicated operation, tedious sample preparation, etc., and achieve the effects of good selectivity, simple detection process and accurate results.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1: Cu 2+ Preparation of the sensor:
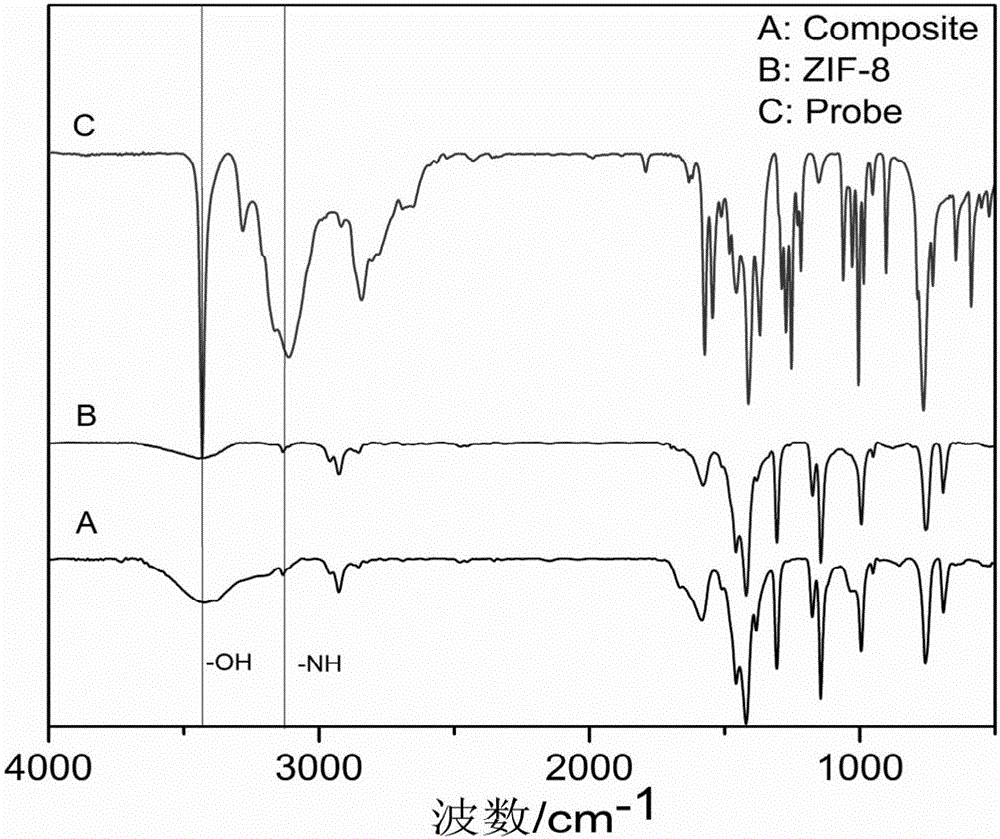
[0024] (1) The preparation steps of MOF nanomaterials are: weigh Zn(NO) 3 ·6H 2 O 0.298g and 2-methylimidazole 0.246g were dissolved in 15mL of methanol respectively, then the two were mixed and transferred to a 100mL hydrothermal reaction kettle, reacted at a constant temperature of 80°C for 24h, and washed with methanol for 3 times, 100 °C and vacuum dried for 24 hours to obtain ZIF-8 solid powder.
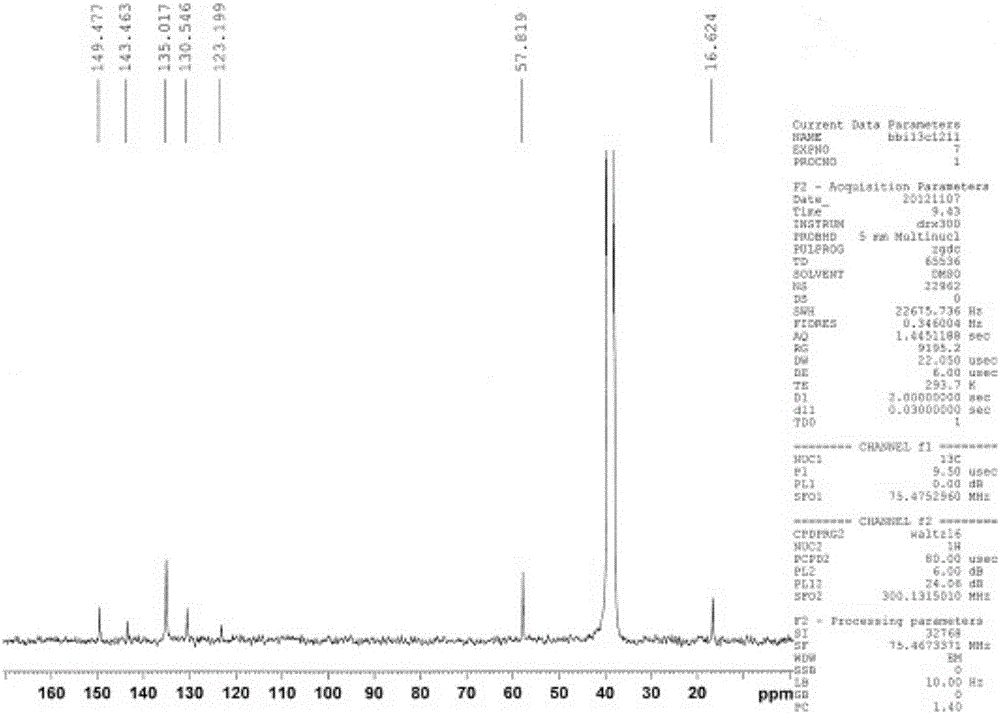
[0025] (2)Cu 2+ Preparation of fluorescent probe: Weigh pyrimidine hydrochloride (C 8 h 9 NO 3 HCl) 0.4072g, fully dissolved in 10mL absolute ethanol, then add 85% hydrazine hydrate (N 2 h 4 ·H 2 (0) 2 ~ 5mL, reflux at 70 ~ 80 ° C for 8 h, cooling, after the crystals are precipitated, filter, wash, and then vacuum dry at 60 ° C for 24 h to obtain a solid powder, its nuclear magnetic spectrum is as follows figure 1 . The stoichiometric ratio of described pyridoxal hydrochloride and hydrazine hydrate is 1:1.
[0026]...
Embodiment 2
[0027] Example 2: Fluorescent Probes and Sensors on Cu 2+ Applications in testing
[0028] (1) Preparation of fluorescent probe solution: Accurately take 18.1 mg of fluorescent probe, dissolve it with 80 mL of absolute ethanol, transfer it to a 100 mL volumetric flask, and prepare a concentration of 1.0×10 -3 mol / L fluorescent probe stock solution (stored at a constant temperature at 4°C).
[0029] (2) Preparation of the new sensor stock solution: Take 100 mg of the new sensor, dissolve it in 90 mL of solvent (deionized water: absolute ethanol = 8:1), transfer it to a 100 mL volumetric flask to constant volume (keep it at a constant temperature at 4°C) ).
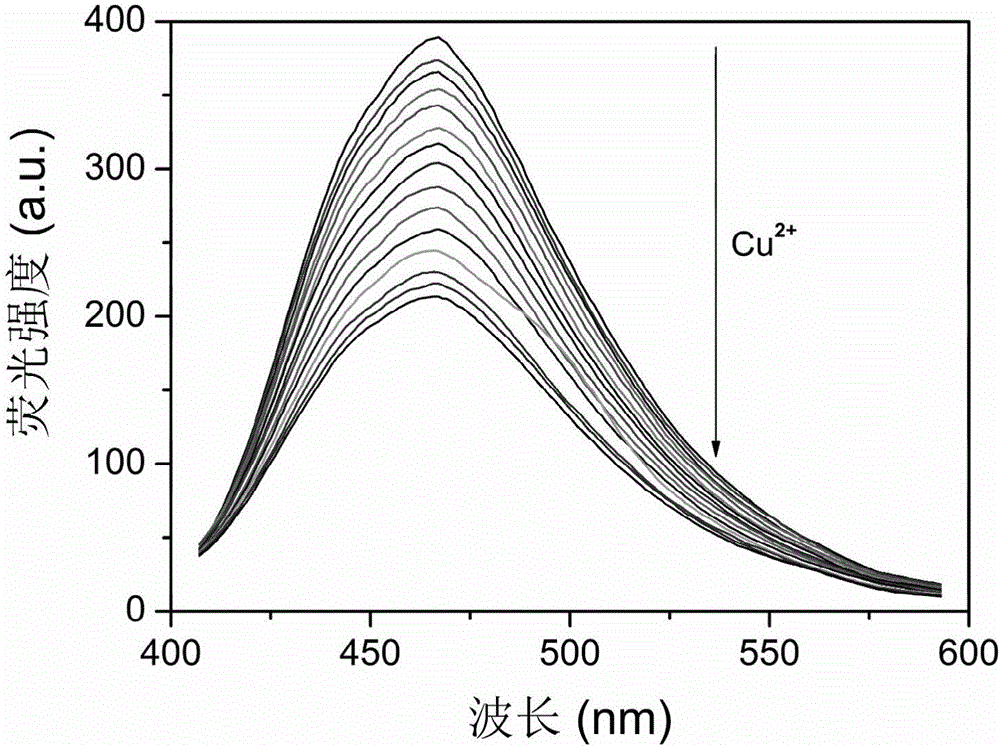
[0030] (3) Fluorescent probes for Cu 2+ Detection: the wavelength of the excitation light is set to 358nm, the wavelength of the emission light is set to 471nm, the slit of the excitation light source is 10.0nm, the slit of the emission light source is 10.0nm, and the test condition is normal temperature. Take 3mL of 0....
Embodiment 3
[0032] Embodiment 3: novel sensor pair Cu 2+The wavelength of the excitation light is set to 358nm, and the wavelength of the emission light is set to 471nm. Take 3mL of 0.01mol / L PBS buffer solution with pH=9 and 20μL of 1.0g / L new sensor stock solution and add them to 15 clean fluorescence ratios respectively. In the color dish, add the same amount of Cu in turn 2+ , Cr 3+ , Hg 2+ ,Co 2+ , Ni 2+ , Al 3+ , Zn 2+ , Mn 2+ , Ba 2+ , Mg 2+ , Pb 2+ , Cd 2+ , Ca 2+ , Ag + and Fe 3+ , the concentration is 2.0×10 -3 mol / L, measure the fluorescence intensity values respectively, and draw the fluorescence intensity histograms corresponding to different metal ions with the fluorescence intensity as the ordinate, as shown in Figure 7 shown. Other common metal ions do not interfere with the system for the determination of copper ions.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com