Antibacterial peptide Enterocin P and preparation method and application thereof
A technology of antimicrobial peptides and host bacteria, applied in the field of genetic engineering, to achieve strong antibacterial activity, no antibiotic resistance, good effect and probiotic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] The preparation method of the antimicrobial peptide Enterocin P of the present invention, comprises the steps:
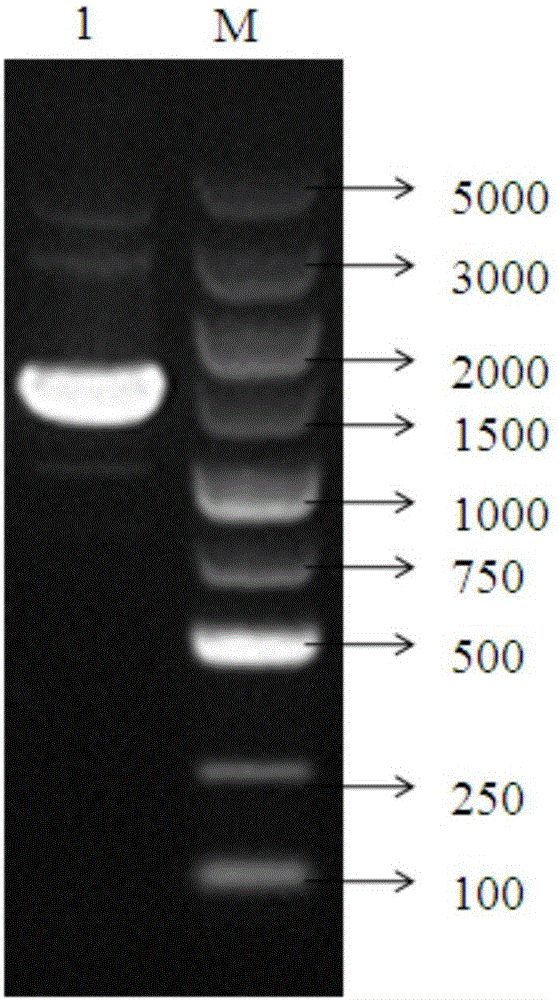
[0050] (1) The gene whose nucleotide sequence is artificially synthesized as shown in SEQ ID NO: 3; the artificially synthesized Enterocin P gene with restriction sites containing Sac I and Hind III on both sides has the nucleotide sequence shown in SEQ ID No.3 .
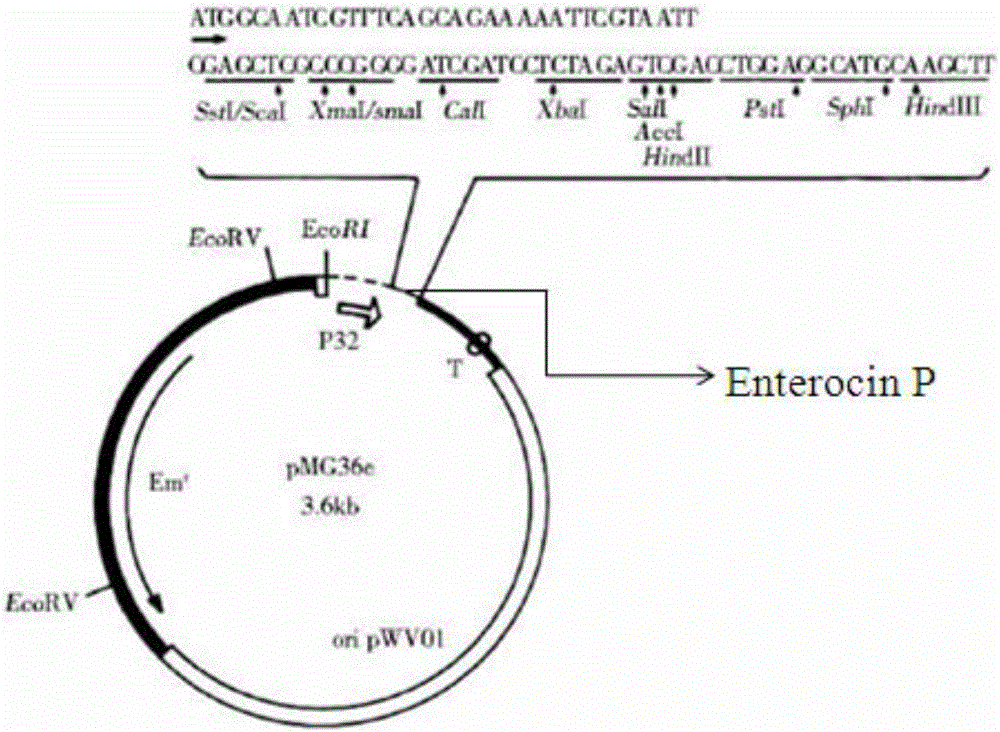
[0051] (2) Construct the pMG36e-Enterocin P recombinant plasmid capable of highly expressing the antimicrobial peptide Enterocin P; connect the nucleotide sequence shown in SEQ ID No.3 with restriction endonucleases Sac I and Hind III and linearize after double digestion The pMG36e plasmid was ligated to obtain the pMG36e-Enterocin P ligation product, and the ligation product was introduced into Escherichia coli DH5α to obtain a recombinant plasmid containing pMG36e-Enterocin P.
[0052] (3) using the pMG36e-Enterocin P recombinant plasmid to transform the host bacterium to construct a genetically...
Embodiment 1
[0059] Main material used in the present invention
[0060] Strains and vectors: Escherichia coli MC1061, Lactococcus lactis MG1363 and pMG36e plasmid bacteria were purchased from Mo Bi Tec, Germany; DNA Marker was purchased from Shanghai Jierui Co., Ltd.; Listeria, Staphylococcus aureus and Salmonella were preserved in our laboratory ; The antibacterial peptide Enterocin P gene was synthesized by Sangon Bioengineering (Shanghai) Co., Ltd. and transferred into Escherichia coli DH5α; primers were synthesized by Sangon Bioengineering (Shanghai) Co., Ltd.
[0061] Main reagents and kits: M17 broth medium was purchased from Qingdao Haibo Company; restriction enzymes Sac Ⅰ and HindⅢ, T4 DNA Ligase, EX Taq were purchased from TaKaRa Biotech Company; erythromycin, plasmid extraction and DNA gel The recovery kit was purchased from Tiangen Company; nisin was purchased from Sigma Company.
[0062] Preparation of main reagents:
[0063] GM17: M17 broth medium was autoclaved at 121° C. ...
Embodiment 2
[0076] Preparation of Escherichia coli DH5α Competent Cells
[0077] 1. 1% LB liquid medium inoculated with DH5α, shake overnight at 37°C;
[0078] 2. Take 1% of the overnight culture solution and inoculate it in LB liquid medium, culture it with shaking at 37°C for 4 hours (the bacteria are in the logarithmic growth phase at this time), and place it on ice for 10 minutes;
[0079] 3. Centrifuge at 4000g for 10min at 4°C to collect the logarithmic phase cells, discard the supernatant, and collect the bacterial cell pellet;
[0080] 4. Use pre-cooled 0.1M CaCl 1 / 10 of the volume of the previous liquid medium 2 Bacteria, placed on ice for 30 minutes;
[0081] 5. Centrifuge at 4000g for 5 minutes at 4°C, discard the supernatant, and collect the bacterial cell pellet;
[0082] 6. Use 1 / 100 of the volume of the previous liquid medium containing 0.1M CaCl 2 Solution resuspended bacteria;
[0083] 7. Pipette 200 μL into centrifuge tubes and store at -80°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com