A calixarene-based 1,3,4-oxadiazole cu 2+ Fluorescent probe and its synthesis method
A fluorescent probe, oxadiazole technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve problems such as poor practicability, and achieve the effect of short synthesis route, high sensitivity and excellent stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Synthesis of intermediate compound 2a
[0027] Add 1a (0.33g, 1.4mmol), benzoyl peroxide (17mg, 70μmol), N-bromosuccinimide (130mg, 0.75mmol), 25mL carbon tetrachloride respectively in a 50mL round bottom flask , stirred, heated to reflux for 2h, then added N-bromosuccinimide (130 mg, 0.75mmol), and continued to heat to reflux for 3h. After cooling, the solvent was distilled off under reduced pressure, 25 mL of dichloromethane was added, the resulting solution was washed with saturated brine and distilled water, and dried over anhydrous magnesium sulfate for 1 hour. Filtration, solvent removal by distillation under reduced pressure, thick product is purified by silica gel column chromatography (eluent: V 乙酸乙酯 :V 石油醚 =1:15), to obtain 2a as a white solid. Yield 85%.
Embodiment 2
[0029] With Ia(R 1 =(CH 3 ) 3 C, R 3 =H) as an example to illustrate the synthesis of fluorescent probe molecule I
[0030] In a 25 mL round bottom flask was added 3a (195 mg, 0.3 mmol), anhydrous Na 2 CO 3 (71mg, 0.7mmol) and 10mL of anhydrous acetone, magnetically stirred, heated to reflux for 1h. After cooling slightly, 2a (190mg, 0.6mmol) was added and heated to reflux for 24h. Cool to room temperature, remove the solvent by distillation under reduced pressure, add 10 mL of water to the residue, extract three times with dichloromethane (3×10 mL), combine the organic phases, wash with saturated brine and distilled water successively, and dry over anhydrous magnesium sulfate for 1 hour . Filtration, solvent removal by distillation under reduced pressure, thick product is purified by silica gel column chromatography (eluent: V 乙酸乙酯 :V 石油醚 = 1:3), to obtain Ia as a white solid. Yield 46%.
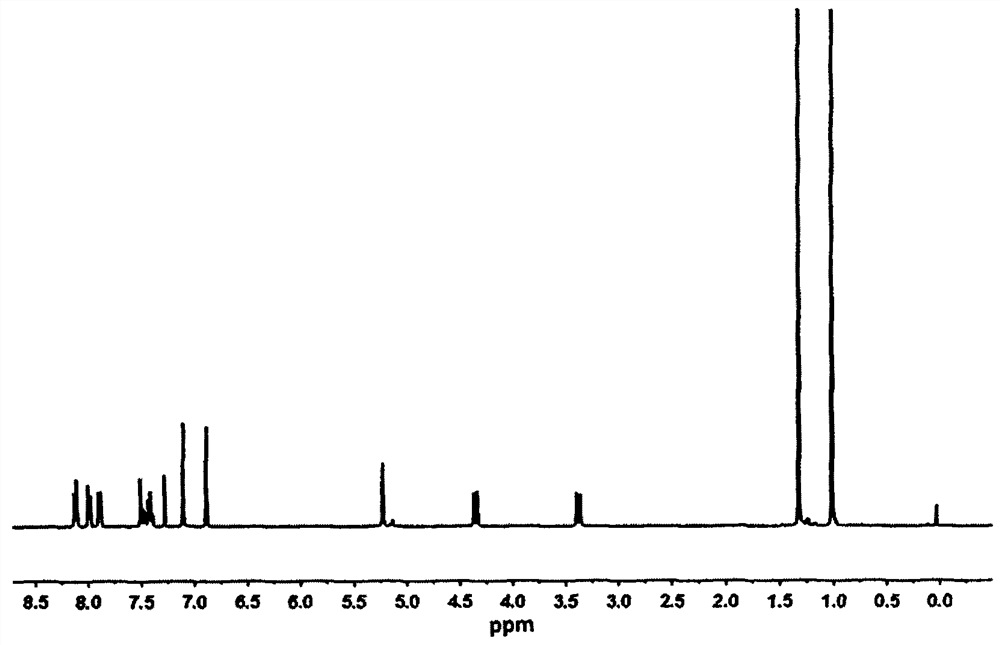
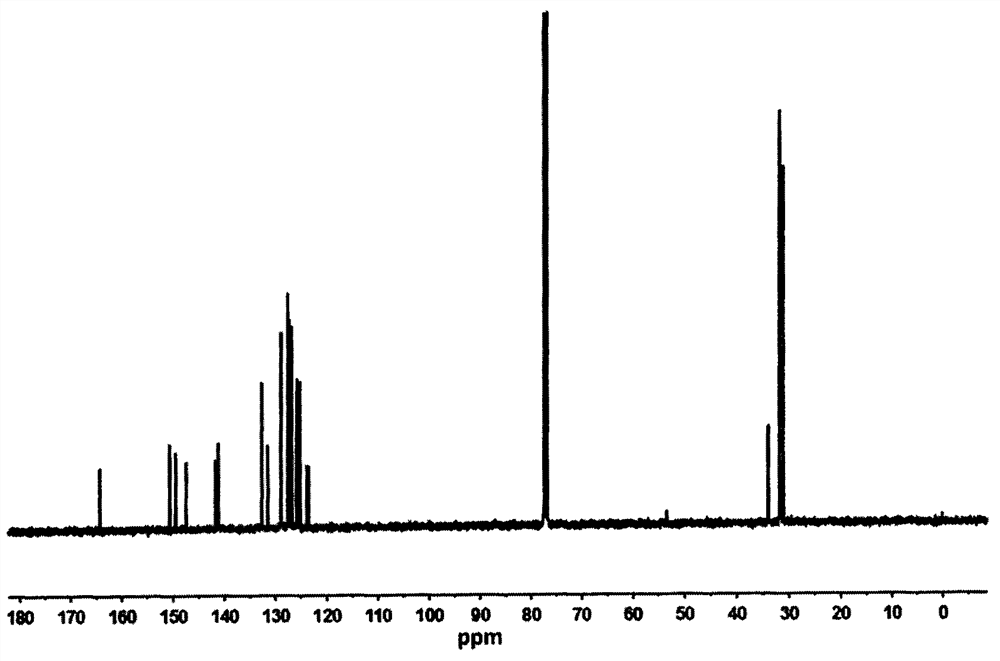
[0031] Such as figure 1 Shown, the nuclear magnetic resonance data proton...
Embodiment 3
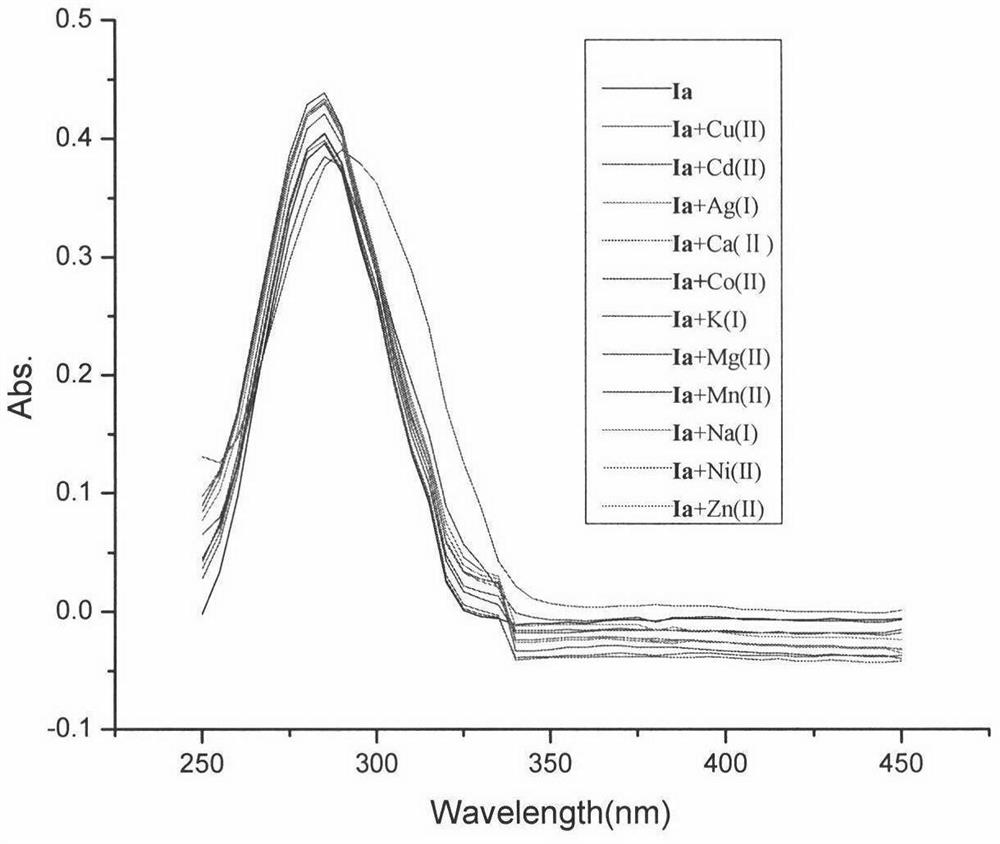
[0035] The ultraviolet-visible absorption spectra of Ia and dichloromethane / acetonitrile (volume ratio 125:1) solution added with different metal ions were tested. The specific method is to prepare a concentration of 8 × 10 -6 The dichloromethane solution of the Ia of mol / L and the Na that concentration is 0.01mol / L + , K + , Ca 2+ , Mg 2+ , Cd 2+ , Ni 2+ ,Co 2+ , Zn 2+ , Ag + , Mn 2+ , Cu 2+ A solution of perchlorate in acetonitrile. Take 2.5 mL of the dichloromethane solution of Ia and add 20 μL of acetonitrile solutions of different metal ions respectively, and test the ultraviolet-visible spectrum after shaking.
[0036] Such as image 3 Shown, the maximum absorption wavelength of the dichloromethane solution of Ia is 285nm, when adding Cu 2+ Finally, the maximum absorption wavelength of Ia is 290nm, resulting in a red shift phenomenon, and the absorbance also decreases obviously, while adding other ions, the maximum absorption wavelength and absorbance do not...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com