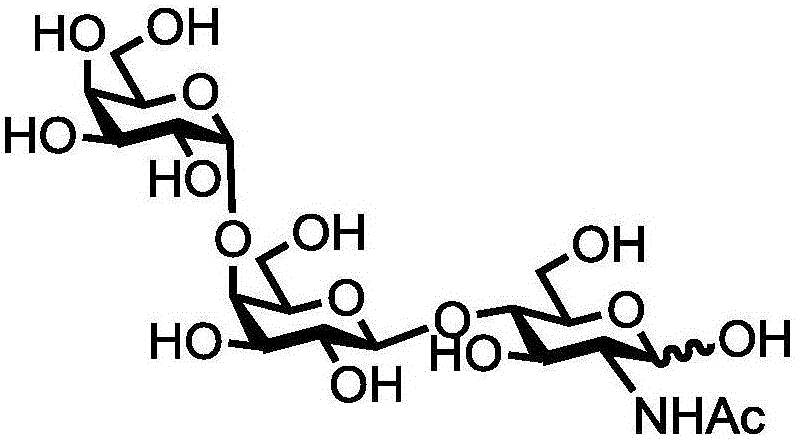
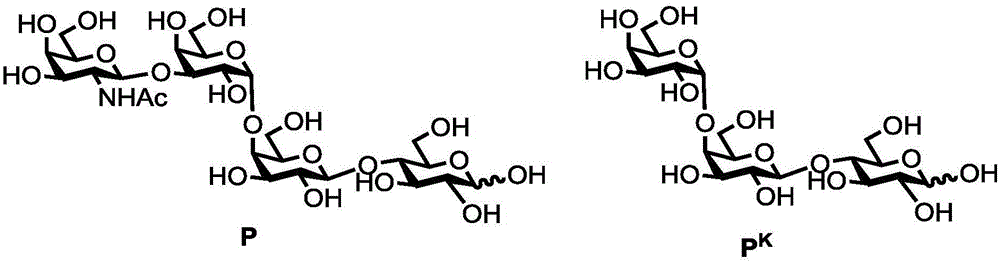
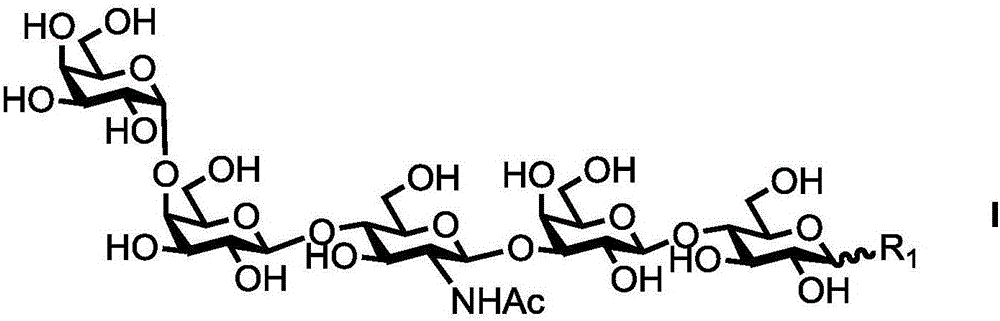
Human blood group antigen P1 pentasaccharide synthesis method
A synthesis method and blood group antigen technology, which are applied in the field of synthesis of human blood group antigen P1 pentasaccharide, can solve the problems of low overall yield, many reaction steps, uneven products, etc., and achieve low reaction efficiency, few synthesis steps, and rapidity. synthetic effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Example 1: Synthesis of P1 Antigen Pentasaccharide
[0048] Proceed as follows:
[0049] (1) Chemical synthesis of β-configuration lactose compound 1 (Galβ1,4GlcβProN 3 )
[0050] Lactose (10 g, 29.23 mmol), acetic anhydride (55 mL) and sodium acetate (9.6 g) were added to a 500 mL round bottom flask, and stirred under reflux at 160° C. for 6 hours. Thin layer chromatography (PE: EA = 1: 1) after the reaction was complete, concentrated by rotary evaporation. The resulting solid was redissolved in 300 mL of dichloromethane, extracted once with half-saturated saline, extracted three times with saturated sodium bicarbonate solution, extracted three times with double-distilled aqueous solution, then separated the organic phase, dried the organic phase with anhydrous sodium sulfate, and rotary evaporated Concentration afforded Compound 5 (18.6 g, 94%) as a light yellow solid.
[0051] Add compound 5 (1.0 g, 1.47 mmol), dichloromethane (5.0 mL), boron trifluoride diethyl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com