A method for enzymatically preparing n-bromoacetyl-7-aminocephalosporanic acid
An aminocephalosporanic acid, catalytic preparation technology, applied in the direction of fermentation, etc., can solve the problems of complicated operation, unsuitable for sustainable social and environmental development, harsh conditions, etc., achieves simple and easily controllable reaction process, realizes reuse, and reaction conditions mild effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Add 10mL phosphate buffer (100mM, pH 7.5), 40mM 7-ACA and 120mM methyl bromoacetate into a 50mL Erlenmeyer flask with a cap, mix well, then add 30U penicillin G acylase II (purchased from CSPC), React at 20°C and 200r / min. After 11 h, liquid chromatography analysis showed a 34% yield of N-bromoacetyl-7-ACA.
Embodiment 2
[0031] Add 10mL phosphate buffer (100mM, pH 7.5), 40mM 7-ACA and 120mM methyl bromoacetate into a 50mL Erlenmeyer flask with a cap, mix well, then add 30U penicillin G acylase IV (purchased from CSPC), React at 20°C and 200r / min. After 7 h, liquid chromatography analysis showed that the yield of N-bromoacetyl-7-ACA was 32%.
Embodiment 3
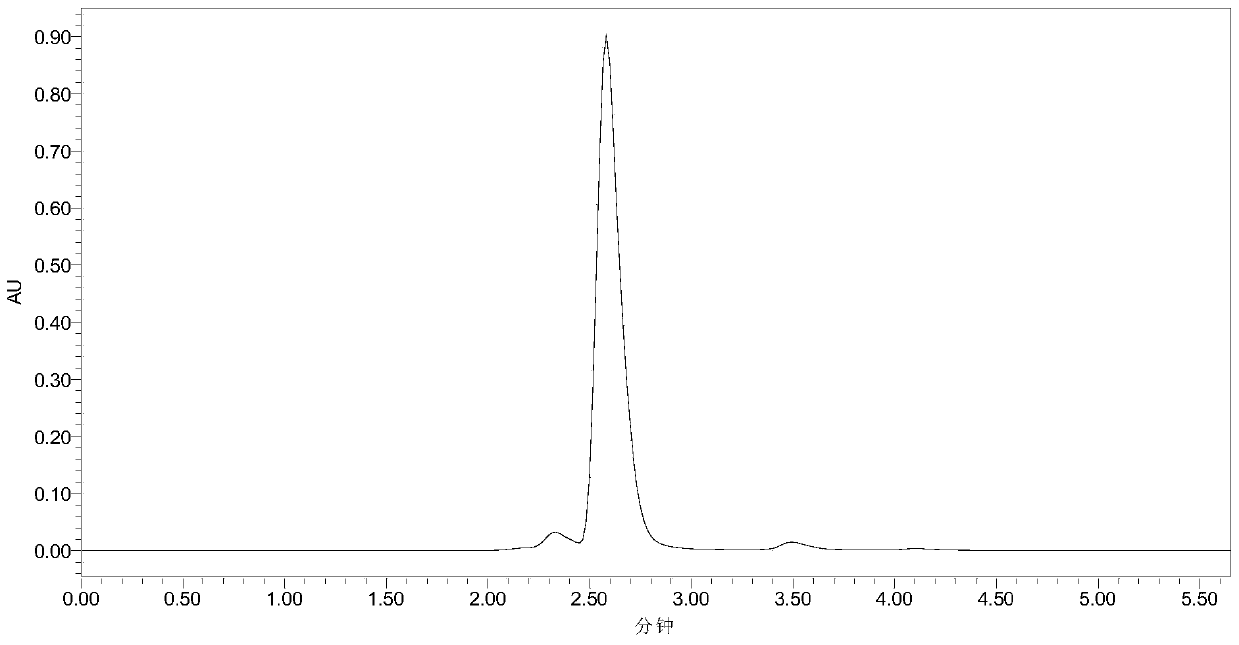
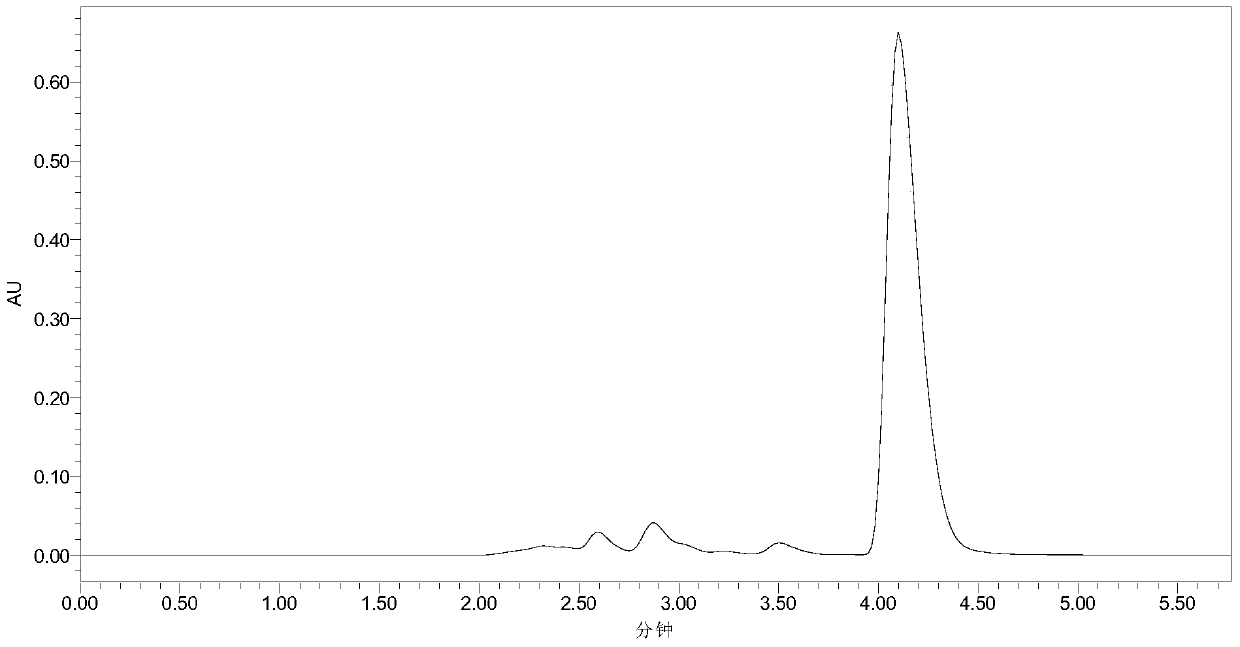
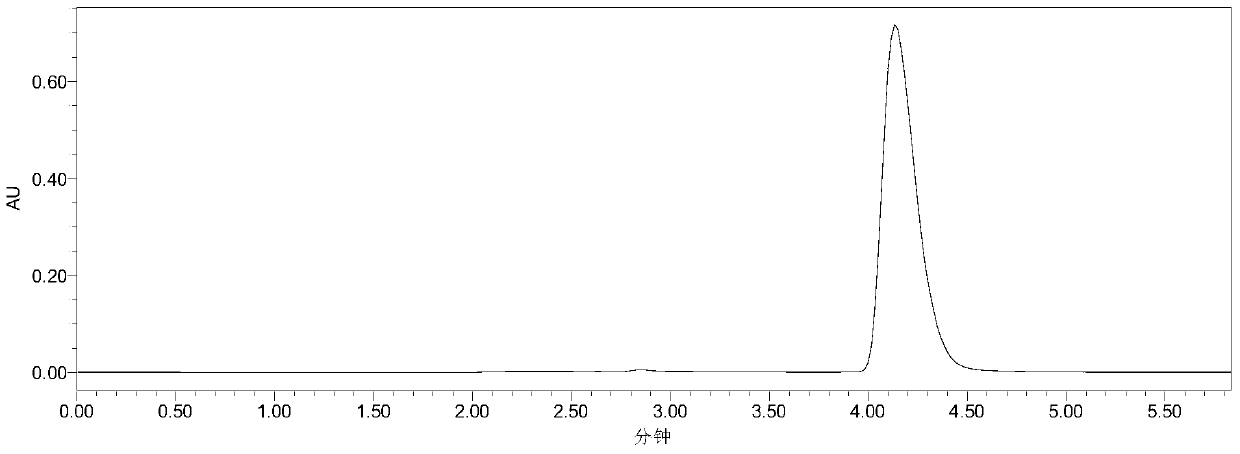
[0033] Add 10mL phosphate buffer (100mM, pH 7.5), 40mM 7-ACA and 120mM methyl bromoacetate into a 50mL Erlenmeyer flask with a cap, mix well, then add 30U penicillin acylase PGA-750 (purchased from Zhejiang Shunfeng Haider Co., Ltd.), react at 20°C and 200r / min. After 3 h, liquid chromatography analysis showed that the yield of N-bromoacetyl-7-ACA was 91%. The liquid chromatograms before and after the reaction are shown in the attached figure 1 and attached figure 2 As shown, the retention times of 7-ACA and N-bromoacetyl-7-ACA were 2.58 and 4.10 min, respectively. Subsequently, the enzyme was filtered out, and hydrochloric acid was added to the filtrate to adjust the pH to 1.0, filtered, washed with water, and dried to obtain N-bromoacetyl-7-ACA with a yield of 88%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com