Sucralfate-colloidal bismuth pectin compound pharmaceutical tablets and preparation method thereof
A technology of colloidal bismuth pectin and sucralfate, which can be used in drug combination, pill delivery, and pharmaceutical formulations, can solve the problems of severe gastrointestinal reactions and short half-life, and achieve smooth muscle spasm, inhibit gland secretion, improve Fluidity and solubility effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
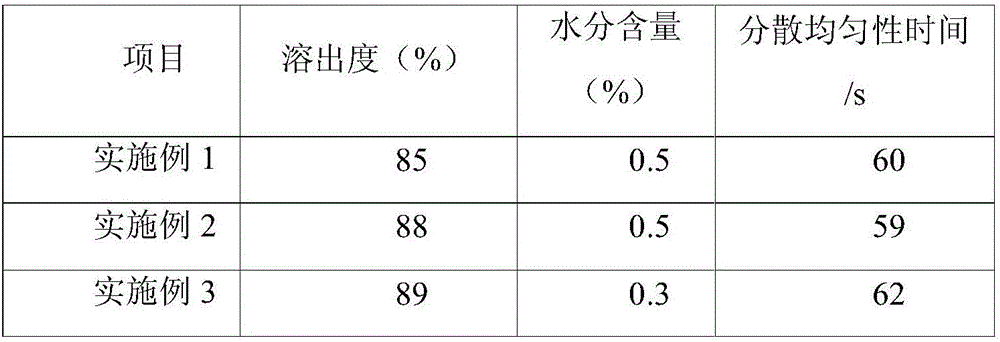
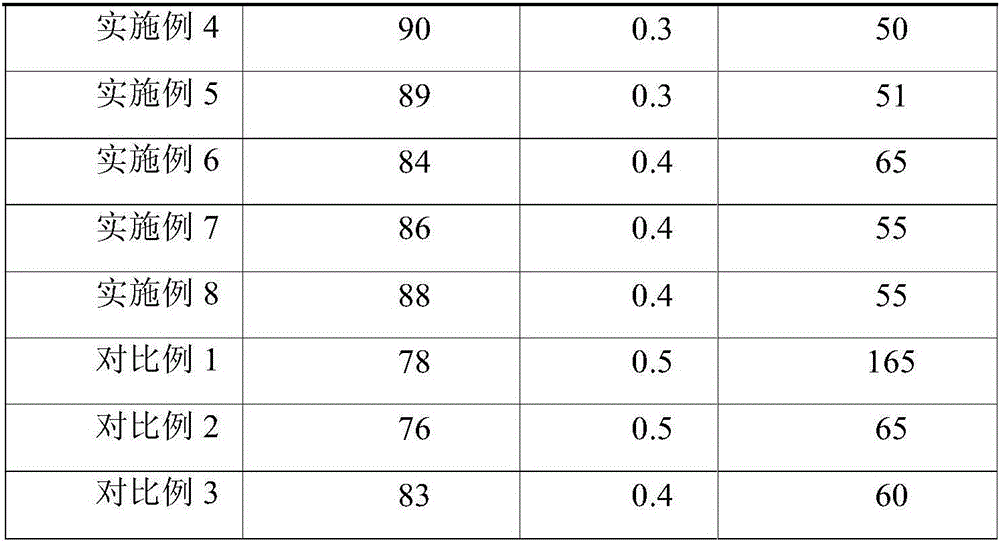
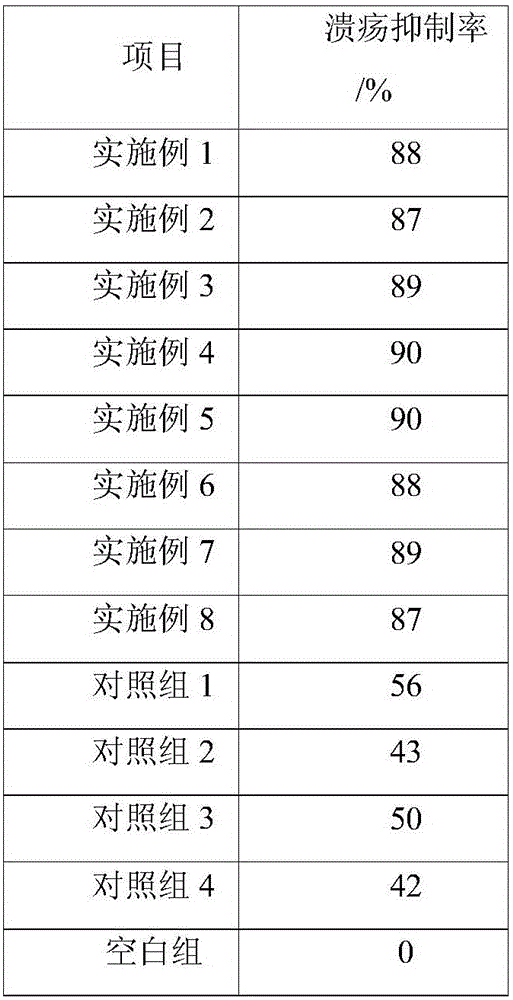
[0031]A sucralfate colloidal bismuth pectin compound drug tablet, comprising the following raw materials in parts by weight: 20 parts of colloidal bismuth pectin as bismuth, 10 parts of sucralfate as aluminum, 30 parts of belladonna extract, and astragalus extract 2 parts, 2 parts of Atractylodes macrocephala extract, 2 parts of Fangfeng extract, 5 parts of Radix Radix Radix, 20 parts of hypromellose phthalate, 3 parts of microcrystalline cellulose, 15 parts of low-substituted hydroxypropyl cellulose 2 parts of magnesium stearate and 0.1 part of glycyrrhizin.
[0032] Its preparation method comprises the following steps:
[0033] 1) Grinding and manufacturing pellet cores: Grinding colloidal bismuth pectin, sucralfate, and belladonna extract, drying, and centrifuging to granulate to obtain the first pellet core. , Radix isatidis extract, pulverized and passed through a 250-mesh sieve, then mixed evenly, and centrifugally granulated to obtain the second pellet core;
[0034] ...
Embodiment 2
[0039] A sucralfate colloidal bismuth pectin compound drug tablet, comprising the following raw materials in parts by weight: 60 parts of colloidal bismuth pectin as bismuth, 20 parts of sucralfate as aluminum, 40 parts of belladonna extract, and astragalus extract 40 parts, 40 parts of Atractylodes macrocephala extract, 40 parts of Fangfeng extract, 80 parts of Radix Radix Radix, 30 parts of hypromellose phthalate, 5 parts of microcrystalline cellulose, 30 parts of low-substituted hydroxypropyl cellulose parts, 3 parts of magnesium stearate and 0.2 parts of glycyrrhizin.
[0040] Its preparation method comprises the following steps:
[0041] 1) Grinding and manufacturing pellet cores: Grinding colloidal bismuth pectin, sucralfate, and belladonna extract, drying, and centrifugally granulating to obtain the first pellet core; 1. The Radix isatidis extract is pulverized and passed through a 300-mesh sieve, then mixed evenly, and centrifugally granulated to obtain the second pel...
Embodiment 3
[0047] A sucralfate colloidal bismuth pectin compound drug tablet, comprising the following raw materials in parts by weight: 35 parts of colloidal bismuth pectin as bismuth, 16 parts of sucralfate as aluminum, 35 parts of belladonna extract, and astragalus extract 30 parts, 30 parts of Atractylodes macrocephala extract, 30 parts of Fangfeng extract, 60 parts of Radix Radix Radix, 25 parts of hypromellose phthalate, 3 parts of microcrystalline cellulose, 20 parts of polyethylene glycol, hard 2 parts of magnesium fatty acid and 0.1 part of stevia.
[0048] Its preparation method comprises the following steps:
[0049] 1) Grinding and manufacturing pellet cores: Grinding colloidal bismuth pectin, sucralfate, and belladonna extract, drying, and centrifugally granulating to obtain the first pellet core; 1. The Radix isatidis extract is pulverized and passed through a 270-mesh sieve, then mixed evenly, and centrifugally granulated to obtain the second pellet core;
[0050] 2) Pre...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size (mesh) | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
| particle size (mesh) | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com