Application of cubic boron nitride as mimetic peroxidase
A technology of boron nitride peroxide and cubic boron nitride, which is applied in the field of chemical detection of biological materials, can solve the problems of non-regeneration, poor tolerance, low catalytic efficiency, etc., and achieves low cost, simple preparation process and high stability. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
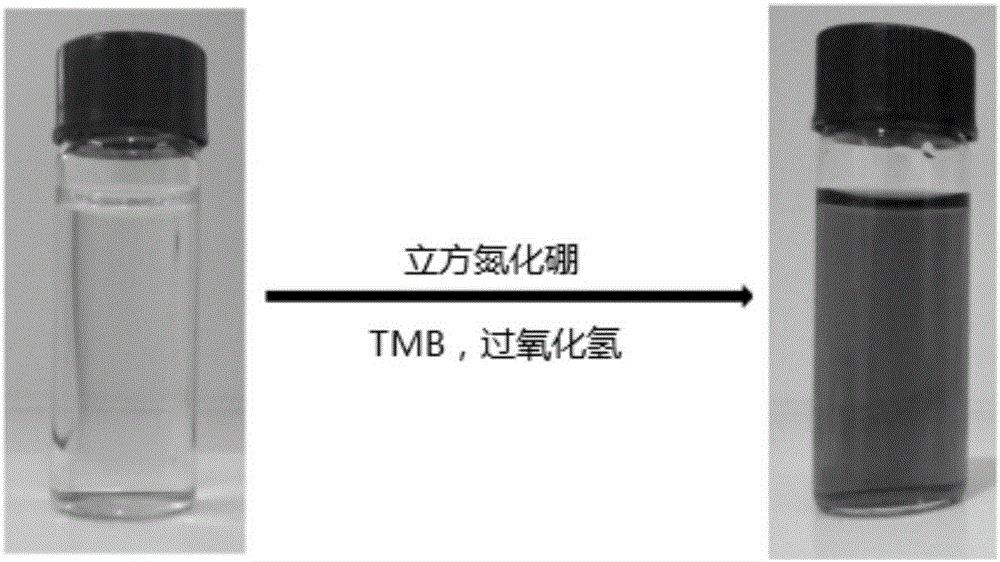
[0042] Weigh 50 mg of cubic boron nitride powder and place it in a centrifuge tube, add 10 ml of ultrapure water and ultrasonically disperse for 10 minutes to prepare a 5 mg / ml cubic boron nitride suspension. Take 3.7ml of citric acid-disodium hydrogen phosphate buffer solution with a pH of 4 and place it in a constant temperature water bath at 45°C for 10 minutes, then add 0.1ml of 3,3',5,5'-tetramethyl Benzidine (TMB) solution, 0.1ml of 10mmol / L hydrogen peroxide solution, and 0.1ml of cubic boron nitride solution, shake well, react at 45°C for 10 minutes, take out, and obtain the sample to be tested after filtration. Using ultrapure water as a blank, measure the absorption spectrum with a UV-Vis spectrophotometer in the wavelength range of 400-800nm.
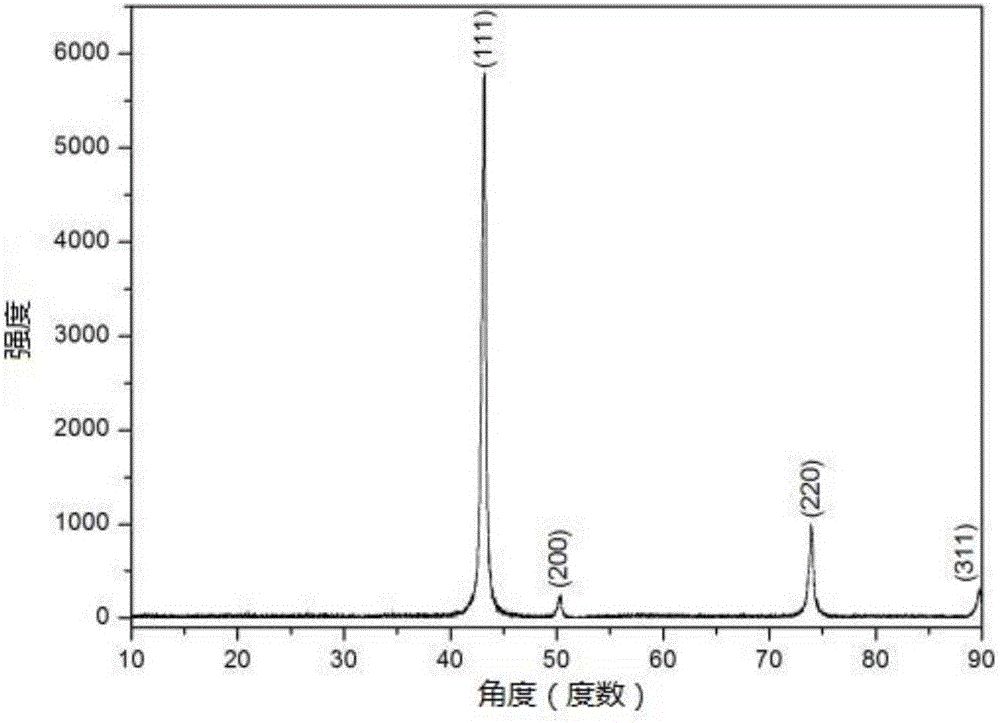
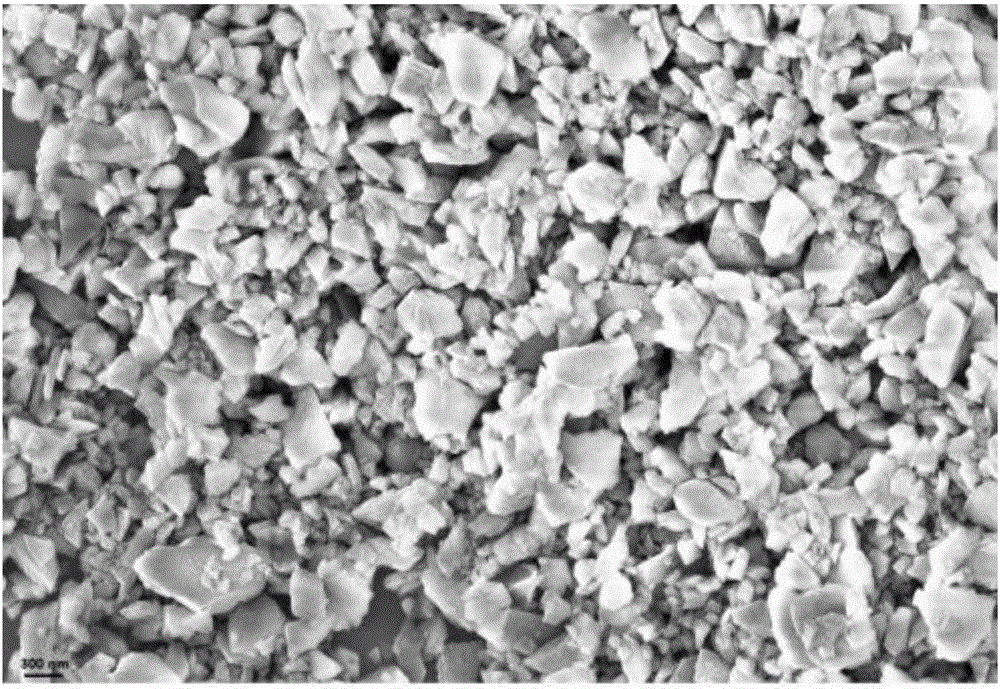
[0043] In addition, in this implementation, X-ray diffraction, scanning electron microscopy and photoelectron spectroscopy analysis and characterization were performed on the above cubic boron nitride.
[0044] The X-ray dif...
Embodiment 2
[0048] Weigh 50 mg of cubic boron nitride powder and place it in a centrifuge tube, add 10 ml of ultrapure water and ultrasonically disperse for 10 minutes to prepare a 5 mg / ml cubic boron nitride suspension. Take 3.7ml of citric acid-sodium hydrogen phosphate buffer solution with pH of 2, 3, 4, 5, 6, 7 and 8 and sodium carbonate-sodium bicarbonate buffer solution with pH of 9, 10 and 11 respectively and place at constant temperature Incubate in a water bath at 45°C for 10 minutes, then add 0.1ml of 1mmol / L 3,3',5,5'-tetramethylbenzidine (TMB) solution and 0.1ml of 50mmol / L hydrogen peroxide solution in sequence , and 0.1ml of cubic boron nitride solution, shake well, react at 45°C for 10 minutes, take out, and obtain the sample to be tested after filtering. Using ultrapure water as a blank, the absorbance at the maximum absorption wavelength was measured with a UV-Vis spectrophotometer.
[0049] The relative peroxidase activity of cubic boron nitride peroxide mimetic enzymes a...
Embodiment 3
[0051] Weigh 50 mg of cubic boron nitride powder and place it in a centrifuge tube, add 10 ml of ultrapure water and ultrasonically disperse for 10 minutes to prepare a 5 mg / ml cubic boron nitride suspension. Take 3.7ml of citric acid-disodium hydrogen phosphate buffer solution with a pH of 4 and place it in a constant temperature water bath for 10 minutes. 3,3',5,5'-tetramethylbenzidine (TMB) solution with a concentration of 1mmol / L, 0.1ml of a 50mmol / L hydrogen peroxide solution, and 0.1ml of a cubic boron nitride solution, shake well , after reacting for 10 minutes at the corresponding temperature, take it out, and obtain the sample to be tested after filtering. Using ultrapure water as a blank, the absorbance at the maximum absorption wavelength was measured with a UV-Vis spectrophotometer.
[0052] The relative peroxidase activity of cubic boron nitride peroxide mimetic enzymes at different temperatures Figure 4b shown.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com