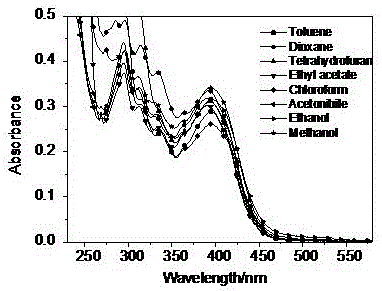
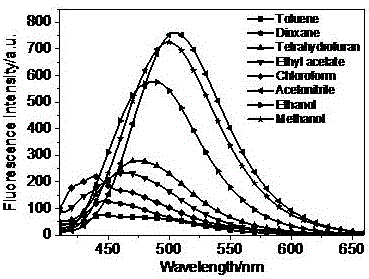
Carbazole-contained benzimidazole-substituted quinoline derivative, preparation method and application thereof
A technology of benzimidazole and derivatives, applied in the field of analytical chemistry, can solve the problems of low detection limit, probe selectivity interference, etc., and achieve the effect of fast detection process, strong complexation and accurate results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] 3-(2-(8-(1H-Benzo[d]imidazol-2-yl)quinolin-2-yl)vinyl)-9-benzyl-9H-carbazole (zinc ion fluorescent probe)
[0039] Add 0.1g 2-methyl-8-(2-benzimidazolyl)quinoline, 0.17g 9-benzyl-9H-carbazole-3-carbaldehyde, 8mL n-butanol, 1.0mL piperpene Pyridine, 0.5mL glacial acetic acid, refluxed for 5h, cooled, a yellow solid precipitated out, and the solid filtered by suction was recrystallized with ethanol aqueous solution to obtain 0.61g of the product, yield: 75.1%. 1H NMR (400MHz, CDCl3): δ: 13.87(s, 1H), 9.10(d, J=7.1Hz, 1H), 8.41(s, 1H), 8.23-8.14(m, 2H), 7.90(s, 1H ),7.87-7.75(m,3H),7.72(d,J=8.6Hz,1H),7.62(t,J=7.7Hz,1H),7.51-7.38(m,4H),7.37-7.26(m, 6H), 7.16(d, J=6.6Hz, 2H), 5.52(s, 2H); ESI-MS, m / z(%): 527.2633(100)[M+H+].
Embodiment 2
[0041] (1) Preparation of test solution:
[0042] In a 10mL sample bottle, add 1.0mL HEPES buffer solution (pH=7.40), then add 0.1mol / L Zn 2+ Standard solution (20μL, 20eq), then add 9.0mL acetonitrile, mix well; finally add 100μL 1×10 -3 Probe L in ethanol, mix again. After standing for 30 minutes, the ultraviolet absorption and fluorescence emission were measured at 390nm as the excitation wavelength. The above operation, without adding metal ion solution, is the preparation of blank test solution. Measure UV absorption and fluorescence emission.
[0043] (2) Ultraviolet spectrum and fluorescence spectrum test:
[0044] The blank test solution of probe L has a weaker fluorescence intensity at 490nm, and no fluorescence emission at 570nm; when zinc ions exist, the fluorescence intensity at 490nm is significantly weakened, and the fluorescence intensity at 570nm is significantly enhanced, see Figure 8 .
[0045] (3) Ion selectivity experiment:
[0046] When various met...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com