Oxazolidone ring-containing epoxy resin, method for producing the thereof, epoxy resin composition, application and epoxy resin hardener
A technology of oxazolidinone ring and epoxy resin, which is applied in the fields of prepreg, epoxy resin cured product, printed circuit board, and laminated board, and can solve the problem of insufficient dielectric properties and insufficient adhesion, etc. problems, to achieve good properties, low dielectric constant, excellent dielectric properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0102] Although an Example and a comparative example were given and this invention was demonstrated concretely, this invention is not limited to these examples. Unless otherwise specified, "part" means a mass part, and "%" means a mass %. In addition, the measuring method can be measured according to the following methods, respectively.
[0103] Epoxy equivalent: According to JISK7236 standard.
[0104] Viscosity: According to JISK7233 standard, single cylinder rotational viscometer method.
[0105] Softening point: Measured according to JIS K7234 standard and ring and ball method. Specifically, an automatic softening point device (manufactured by Mindac Co., Ltd., ASP-MG4) was used.
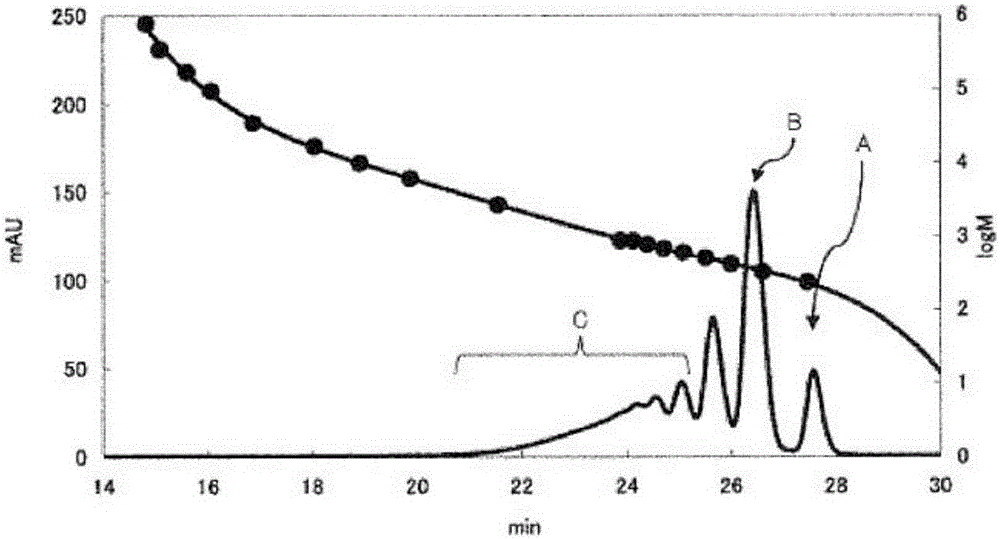
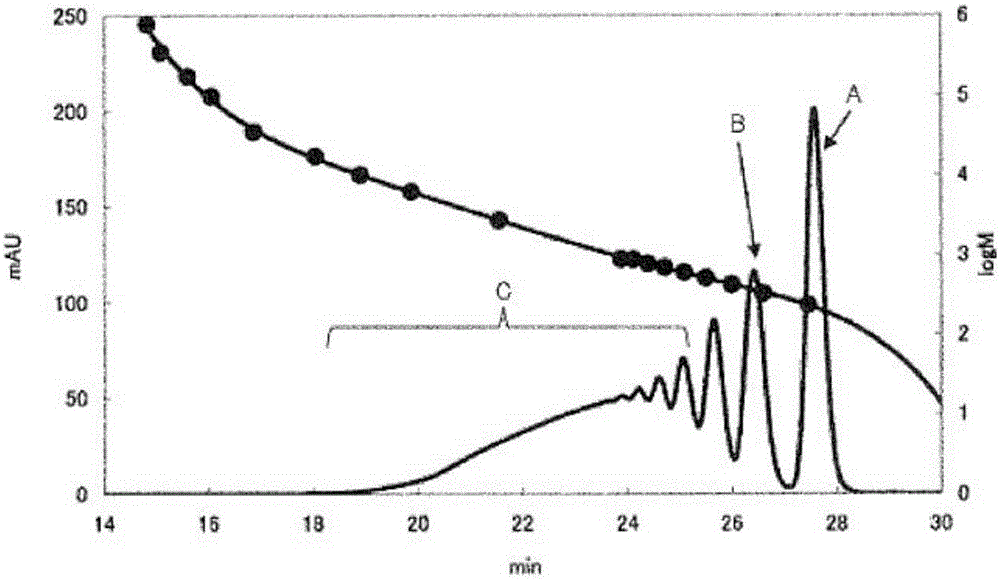
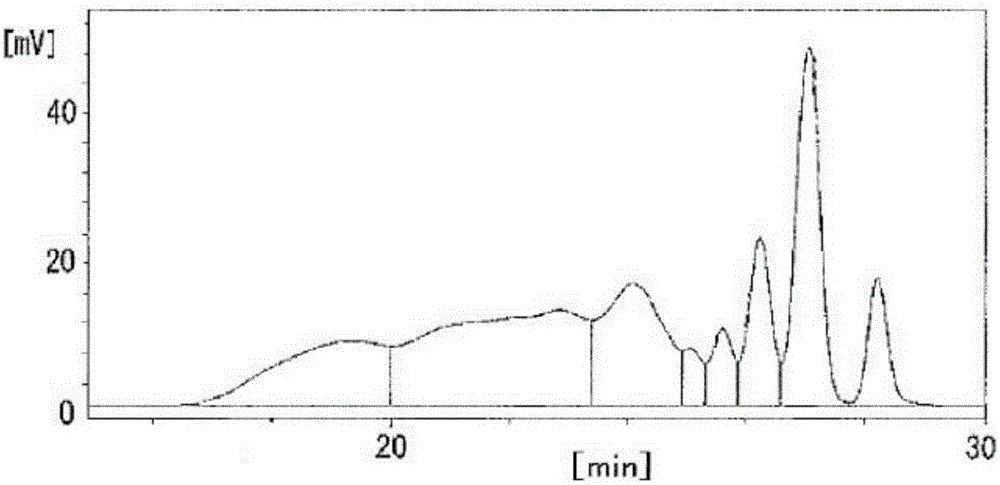
[0106] Dinuclear body content rate, trinuclear body content rate, tetranuclear body content rate, pentanuclear body content rate, number average molecular weight (Mn), weight average molecular weight (Mw), and dispersion (Mw / Mn): using GPC And measure the molecular weight distribution, obtain ...
Synthetic example 1
[0113] Synthesis Example 1 (Synthesis of Phenol Novolak Resin)
[0114] 2500 parts of phenol and 7.5 parts of oxalic acid dihydrates were put in the separable glass flask, and it stirred while injecting nitrogen gas, and heated and raised the temperature. Next, stirring at 80 degreeC, 474.1 parts of 37.4% formalin was dripped and made to react over 30 minutes. Further, reaction was performed for 3 hours while maintaining the reaction temperature at 92°C. The temperature was raised, and the temperature was raised to 110° C. while removing the reaction product water to the outside of the system. Residual phenol was recovered at 160° C. under reduced pressure, and the temperature was further raised to 250° C. to recover a part of dinuclear bodies, thereby obtaining a phenol novolak resin. The dinuclear body content rate and trinuclear body content rate of the obtained phenol novolac resin were measured by GPC, and it was 10 area % and 38 area %, respectively.
Synthetic example 2
[0115] Synthesis Example 2 (Synthesis of Phenol Novolak Type Epoxy Resin)
[0116] 666 parts of the phenol novolac resin obtained in the synthesis example 1, 2110 parts of epichlorohydrin, and 17 parts of ion-exchange water were put into the apparatus similar to synthesis example 1, and it heated up to 50 degreeC, stirring. After uniform dissolution, 14.2 parts of 49% aqueous sodium hydroxide solution was charged and reacted for 3 hours. Next, after raising the temperature to 64°C, reduce the pressure to such an extent that reflux of water occurs, and add 457.7 parts of 49% sodium hydroxide aqueous solution dropwise over 3 hours. , the epichlorohydrin was returned to the reaction vessel, water was removed from the system, and the reaction was performed. After completion of the reaction, the temperature was increased to 70° C. to perform dehydration, and the temperature was set to 135° C. to collect remaining epichlorohydrin. Return to normal pressure, add 1232 parts of methy...
PUM
| Property | Measurement | Unit |
|---|---|---|
| softening point | aaaaa | aaaaa |
| epoxy equivalent | aaaaa | aaaaa |
| dispersity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com