Method for preparing 2,5-dihydroxymethyl furan through 5-hydroxymethyl furfural catalytic transfer hydrogenation
A technology for dimethylolfuran and hydroxymethylfurfural, which is applied in the field of 5-hydroxymethylfurfural catalytic transfer hydrogenation to prepare 2,5-dimethylolfuran, can solve the problem of poor recovery and reuse performance, atomic Low utilization rate, low hydrogen solubility, etc., to achieve strong economic advantages and industrialization prospects, easy separation, recovery and reuse, and avoid the use of exogenous solvents.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
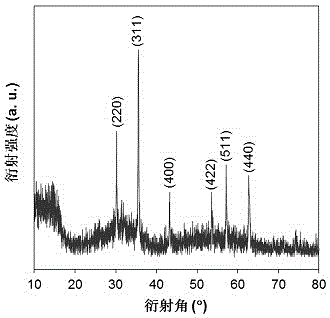
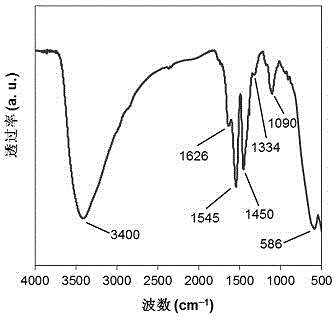
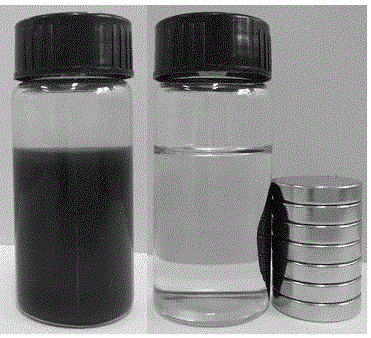
[0018] Example 1: Under a nitrogen atmosphere, nano-ferric ferric oxide is added to a zirconium chloride solution containing 50 g / L prepared with deoxidized deionized water at a molar ratio of iron / zirconium of 1:1, mechanically stirred for 30 min, Ultrasonic dispersion was performed for 10 min; under the condition of a mechanical stirring speed of 200 rpm, ammonia solution was added dropwise to the above solution at a rate of 3 mL / min until pH = 9, stirring was continued for 30 min, and the solution was left to age for 24 h; The solid precipitate was separated by a magnet, and the precipitate was repeatedly washed with deionized water until no chloride ions existed; the washed solid precipitate was vacuum-dried at 105 °C for 12 h, and ground and pulverized to 200 mesh to obtain a magnetic zirconium hydroxide catalyst. Abbreviated as Zr(OH) 4 @Fe 3 o 4 (1:1); Next, 20 g of ethanol, 0.2 g of 5-hydroxymethylfurfural and 0.04 g of Zr(OH) 4 @Fe 3 o 4 (1:1) was added to a 100 ...
Embodiment 2
[0020] Example 2: Under a nitrogen atmosphere, nanometer iron ferric oxide was added to a zirconium oxychloride solution containing 75 g / L prepared with deoxidized deionized water at an iron / zirconium molar ratio of 1:3, and mechanically stirred for 60 min , ultrasonically dispersed for 30 min; under the condition of a mechanical stirring speed of 400 rpm, the ammonia solution was added dropwise to the above solution at a rate of 4 mL / min until the pH = 10, continued stirring for 30 min, and stood for 24 h; The solid precipitate was separated with the help of a magnet, and the precipitate was repeatedly washed with deionized water until no chloride ions existed; the washed solid precipitate was vacuum-dried at 105°C for 12 h, and ground and pulverized to 200 mesh to obtain a magnetic zirconium hydroxide catalyst , referred to as Zr(OH) 4 @Fe 3 o 4 (3:1); Next, 30 g n-butanol, 0.9 g 5-hydroxymethylfurfural and 0.54 g Zr(OH) 4 @Fe 3 o 4 (3:1) was added to a 100 mL Parr auto...
Embodiment 3
[0022] Example 3: Add nano-ferric oxide ferric oxide into a zirconium chloride solution containing 100 g / L prepared with deoxidized deionized water at a molar ratio of iron / zirconium of 1:6 under a nitrogen atmosphere, and mechanically stir for 90 min. Ultrasonic dispersion was performed for 60 min; under the condition of a mechanical stirring speed of 600 rpm, ammonia solution was added dropwise to the above solution at a rate of 6 mL / min until pH = 11, stirring was continued for 30 min, and the solution was left to age for 24 h; The solid precipitate was separated by a magnet, and the precipitate was repeatedly washed with deionized water until no chloride ions existed; the washed solid precipitate was vacuum-dried at 105 °C for 12 h, and ground and pulverized to 200 mesh to obtain a magnetic zirconium hydroxide catalyst. Abbreviated as Zr(OH) 4 @Fe 3 o 4 (6:1); Next, 50 g of isopropanol, 2.5 g of 5-hydroxymethylfurfural and 2.5 g of Zr(OH) 4 @Fe 3 o 4 (6:1) was added t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com