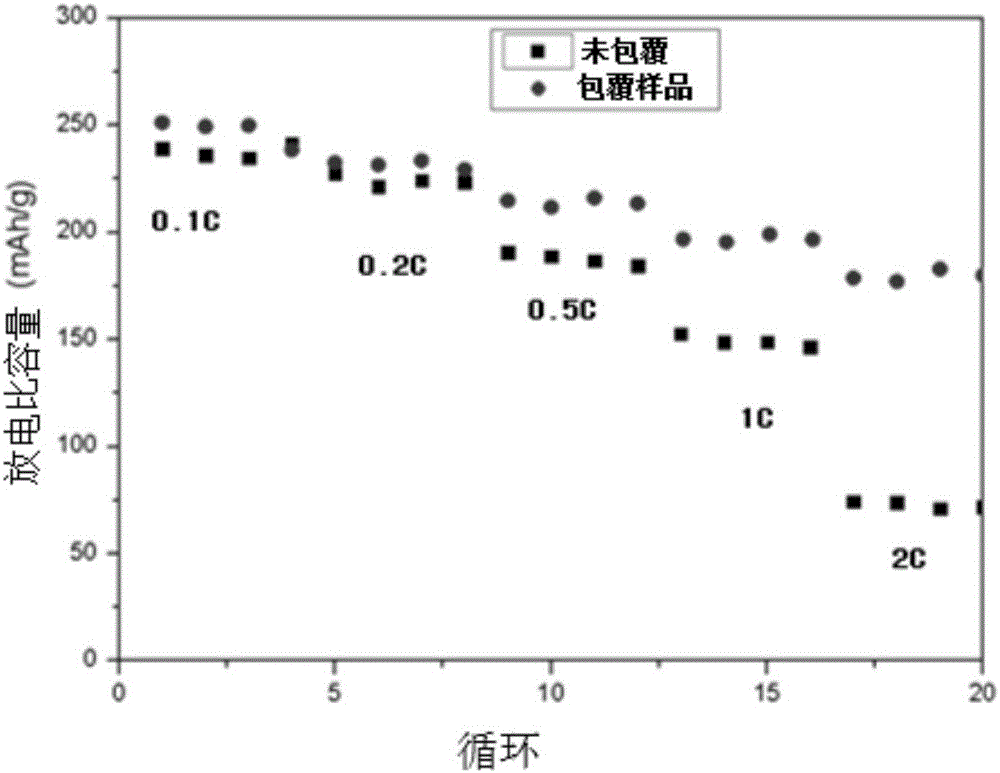
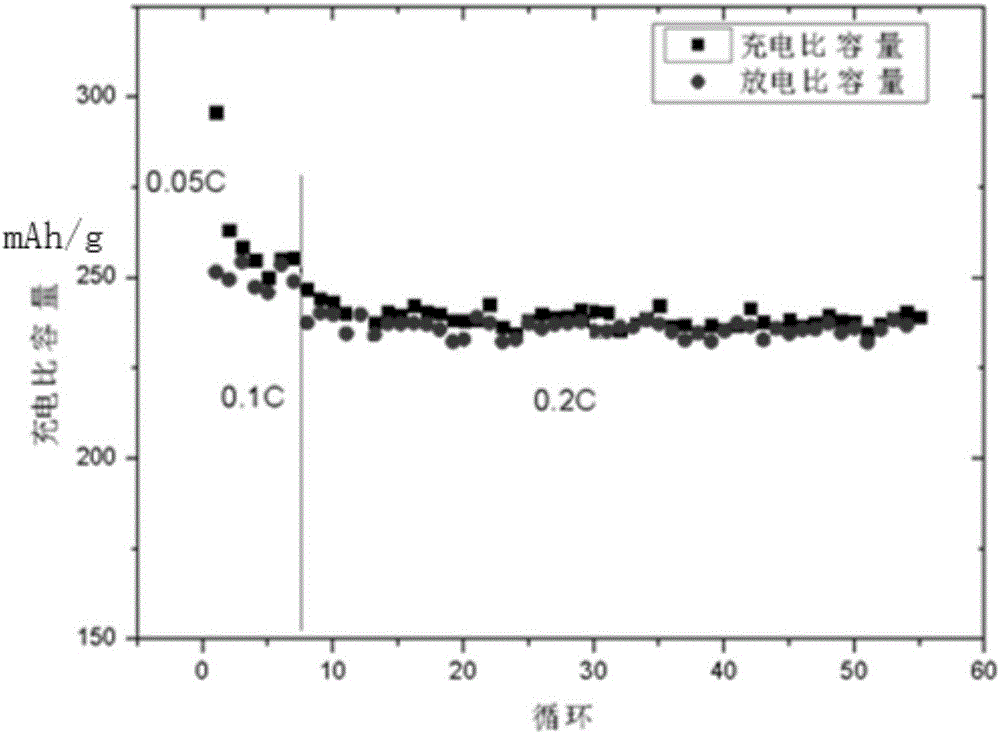
Preparation method of nanometer lithium lanthanum titanate coated 0.5Li2MnO3 0.5LiNi0.5Mn0.5O2 material
A nano-titanium, lithium-coated technology, applied in nanotechnology, nanotechnology, nanotechnology for materials and surface science, etc., can solve the problems of low coulombic efficiency, poor rate performance, capacity decay, etc. Effects of Coulombic Efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] A kind of 0.5Li coated with nanometer lanthanum lithium titanate 2 MnO 3 0.5LiNi 0.5 mn 0.5 o 2 The preparation method of material comprises the following steps:
[0032] (1) Co-precipitation method to prepare nickel-manganese precursor Ni 0.5 mn 1.5 (OH) 4
[0033]Prepare a mixed aqueous solution of nickel nitrate and manganese nitrate at a molar ratio of Ni:Mn=1:3, add 2M NaOH solution dropwise to the mixed aqueous solution under stirring, and control the pH value of the mixed solution at 10.5 to ensure that Ni 2 +, Mn 2+ The precipitation is complete, after standing for 2 hours, suction filtration, washing three times, and drying at 110°C for 12 hours to obtain the precursor Ni 0.5 mn 1.5 (OH) 4 ;
[0034] (2) Nickel-manganese precursor Ni 0.5 mn 1.5 (OH) 4 Preprocessing:
[0035] According to the molar ratio Li: Ni: Mn = 4.1: 1: 3 lithium carbonate, precursor Ni 0.5 mn 1.5 (OH) 4 Mix to obtain a solid mixture, in which a slight excess of Li is to ...
Embodiment 2
[0046] A kind of 0.5Li coated with nanometer lanthanum lithium titanate 2 MnO 3 0.5LiNi 0.5 mn 0.5 o 2 The preparation method of material comprises the following steps:
[0047] (1) Preparation of nickel-manganese precursor Ni by co-precipitation 0.5 mn 1.5 (OH) 4
[0048] Prepare a mixed aqueous solution of nickel nitrate and nickel acetate (the ratio of the two substances is 1:1) and manganese sulfate according to the molar ratio Ni:Mn=1:3, and add molar solution dropwise to the mixed aqueous solution under vigorous stirring. KOH solution with a concentration of 2M, the pH value of the mixed solution is controlled at about 10 to ensure that the Ni 2+ , Mn 2+ The precipitation is complete, after standing for 2 hours, suction filtration, washing three times, and drying at 110°C for 12 hours to obtain the precursor Ni 0.5 mn 1.5 (OH) 4 ;
[0049] (2) Nickel-manganese precursor Ni 0.5 mn 1.5 (OH) 4 preprocessing
[0050] With step 1) gained precursor Ni 0.5 mn ...
Embodiment 3
[0058] A kind of 0.5Li coated on the surface of nanometer lanthanum lithium titanate 2 MnO 3 0.5LiNi 0.5 mn 0.5 o 2 The preparation method of material comprises the following steps:
[0059](1) Preparation of nickel-manganese precursor Ni by co-precipitation 0.5 mn 1.5 (OH) 4
[0060] Prepare a mixed aqueous solution of nickel sulfate, manganese chloride and manganese sulfate (the ratio of the two substances is 1:1) according to the amount ratio of Ni:Mn=1:3, and add it dropwise to the mixed solution under stirring LiOH solution with a molar concentration of 2M, the pH of the mixed solution was controlled at 10.1 to ensure that the Ni 2+ , Mn 2+ The precipitation is complete, after standing for 2 hours, suction filtration, washing three times, and drying at 110°C for 12 hours to obtain the precursor Ni 0.5 mn 1.5 (OH) 4 ;
[0061] (2) Nickel-manganese precursor Ni 0.5 mn 1.5 (OH) 4 For pretreatment, lithium acetate and precursor Ni 0.5 mn 1.5 (OH) 4 Mix the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com